Publication Lists
・Original Papers(2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002)
・Review&Books
2025


●Single Crystal Structures and Photophysical Properties of Brucine Complexes with Axially Chiral Biphenyl Derivatives
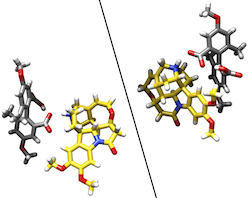
Biphenyl derivatives that are substituted at the 2,2',4,4',6,6'-positions exhibit axial chirality due to restricted rotation around the central bond, rendering them crucial chiral sources for various functional materials. The present study reports the optical resolution and crystal structure determination of the brucine complexes (R)-1•brucine and (S)-1•brucine, and the spectroscopic characterization of (R)-1•brucine, (S)-1•brucine, (R)-1, and (S)-1. The optical resolution was achieved through the diastereomeric complexation of rac-1with brucine, and the absolute configurations of (R)-1•brucine and (S)-1•brucine were confirmed via single-crystal X-ray diffraction analysis. In the complexes, the brucine molecule was tightly bonded to (R)-1 and (S)-1 by the ionic intermolecular hydrogen-bonding interactions. The additional intermolecular CH–π interaction is exclusive to be present between the brucine CH and the aromatic ring of (R)-1, which most likely facilitates the crystallization of (R)-1•brucine over (S)-1•brucine, leading to the efficient optical resolution. The use of UV-vis absorption, fluorescence, and circular dichroism (CD) spectroscopy resulted in distinct spectral signatures arising from a difference in the structures of the intermolecular hydrogen-bonding interactions in these complexes. Time-dependent density functional theory (TD-DFT) calculations employing chloroform solvation models (CPCM) successfully reproduced experimental absorption spectroscopies, thereby demonstrating a charge-transfer HOMO–LUMO electron transition from the brucine unit to the biphenyl unit.
Yudai Ono, Yuma Nakamura, Takeharu Haino, J. Chin. Chem. Soc., 2025, Accepted
●Controlled Molecular Orders in Layered Multiple Porphyrins
Nature precisely regulates multicomponent assemblies with the assistance of cooperativity. However, establishing such high precision in multicomponent assemblies of artificial supramolecular structures remains challenging. Here, we successfully position multiple distinct guest molecules within two equivalent binding cavities of a zinc-metallated trisporphyrin host by combining two distinct negative cooper-ative interactions, including donor–acceptor π-stacking and metal–ligand coordination. Comprehensive characterization using UV-Vis absorption spectroscopy and diffusion-ordered NMR spectroscopy confirmed the exclusive formation of a ternary supramolecular com-plex. X-ray crystallographic analysis further revealed that the introduction of an additional bridging ligand effectively linked the two ternary complexes to produce an unprecedented septenary supramolecular assembly with alternating guest sequences. In contrast to conventional methods, which require structural differentiation or positive cooperativity, our strategy relies exclusively on negative cooperativity to achieve highly precise molecular ordering. This study presents a novel approach toward constructing sophisticated multicomponent molecular assemblies, emphasizing the significant but underutilized role of negative cooperativity in achieving molecular precision in artificial supramolecular chemistry.
Tomoki Kodama, Naoyuki Hisano, Shin-ichi Tate, Takeharu Haino, J. Am. Chem. Soc., 2025, 147, 30674-30683.
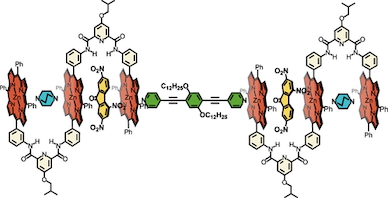
●Vortex-Flow-Directed Chiral Macroscopic Ordering of Platelet Nanostructures Formed via the Supramolecular Assembly of Platinum Complexes with Bis(phenylisoxazolyl)benzene
To understand the vortex flow-directed circular dichroism (CD) effect observed in homogeneous solutions containing supramolecular structures, the macroscopic order formed by supramolecular structures oriented within a flow must be visualized. In this study, a bis(phenylisoxazolyl)benzene-attached platinum complex was found to self-assemble to form uniform anisotropic platelet nanostructures that are oriented within a flow, thereby generating a chiral macroscopic order that is responsible for CD and linear dichroism (LD) effects only in the vortex flow regime. Cooperative self-assembly of a bis(phenylisoxazolyl)benzene-attached platinum complex via controlled supramolecular polymerization produced anisotropic platelet nanostructures with a narrow polydispersity index. The orientational order parameter of the nanostructures correlated with the flow velocity; thus, the nanostructures were oriented along the flow direction. Furthermore, the vortex flow of the dilute nanostructure solution broke the symmetry of the flow, thereby generating a chiral macroscopic order. As a result, CD and LD effects were observed in the vortex flow regime of the dilute nanostructure solution. These results can be generalized to the formation of chiral macroscopic order in solutions containing anisotropic nanostructures.
Masaya Yoshida, Kyota Yasuda, Takuma Matsumoto, Yudai Ono, Naoyuki Hisano, Mao Kawasaki, Takehiro Hirao, Ye Yuan, Shin-ichi Tate, Martin Vacha, Takeharu Haino, J. Am. Chem. Soc., 2025, 147, 30674-30683.
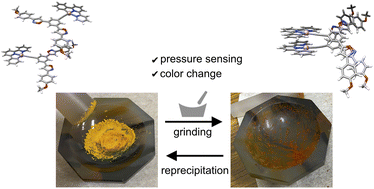
●Mechano-responsive color changes of a Pt(ii) complex possessing triethylene glycol towards pressure sensors
Mechanochromic molecules have attracted significant attention owing to their potential in the development of pressure sensors. However, relatively few studies have investigated the detailed mechanisms of the mechano-responsive nature and the quantitative visualization of mechanical forces. Herein, we report a square-planar platinum complex possessing triethylene glycol chains that exhibits mechanocromic behavior in the amorphous phase. Its mechanochromic nature was established using a combination of spectroscopic techniques, powder X-ray diffraction analyses, and computational chemistry techniques. The continuous changes in emission intensity allowed the platinum complex to be used as a mechanical force sensor, where the output signals were readable using a luminescence spectrometer. These findings demonstrate the potential benefits of square-planar platinum complexes and triethylene glycol chains for the creation of mechanochromic material.
Masaya Yoshida, Takehiro Hirao, Shin-ichi Kihara, Takeharu Haino, RSC Adv., 2025, 15, 21401–21407
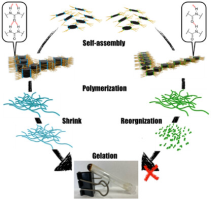
●Supramolecular Aggregates of Amide- and Urea-Functionalized Nanographene
Controlling the morphology of supramolecular nanographene(NG) aggregates is challenging. This study confirms that amideand urea-functionalized NG undergo self-assembly to form supramolecular aggregates with a morphology that depends on the incorporated functional group. Amide-functionalized NG forms stacked aggregates, whereas urea-functionalized NG organizes into network polymers. These distinct morphologies suggest that amide groups drive NG stacking, whereas urea groups support NG vertically and horizontally, likely owing to differences in the strengths of single and bifurcated N─H/O hydrogen bonds. Moreover, the functional group incorporated into NG influences the gelation properties of the system. Among the two tested systems, only urea-functionalized NG formed organogels, possibly because urea–urea hydrogen bonds, enable solvent-molecule trapping inside the network polymers formed in these NG systems. Thus, hydrogen bonds can regulate the morphology and function of supramolecular NG aggregates.
Haruka Moriguchi, Ryo Sekiya, Takeharu Haino, ChemistryEurope, 2025, 3, e2500015.
doi.org/10.1002/ceur.202500015
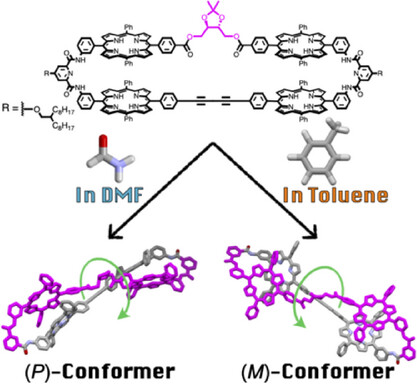
●Solvent-Directed Handedness in a Chirally Twisted Tetrakisporphyrin Macrocycle
A chirally twisted tetrakisporphyrin macrocycle was synthesized by incorporating a chiral dioxolane into a tetrakisporphyrin macrocycle. The solvent type influenced the preferred handedness of the twisted conformation. Circular dichroism measurements and computational analyses determined the handedness of the conformers in solvents toluene and dimethylformamide.
Kouta Tanabe, Naoyuki Hisano, Takeharu Haino, Asian J. Org. Chem. 2025, 14, e00251.
doi.org/10.1002/ajoc.202500251
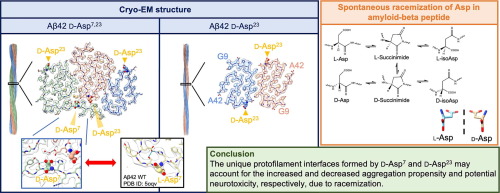
●Impacts of D-aspartate on the Aggregation Kinetics and Structural Polymorphism of Amyloid β Peptide 1–42
Isomerization of L-Aspartate (L-Asp) into D-aspartate (D-Asp) occurs naturally in proteins at a rate that is much faster than that of other amino acid types. Accumulation of D-Asp is age-dependent, which could alter protein structures and, therefore, functions. Site-specific introduction of D-Asp can accelerate aggregation kinetics of a variety of proteins associated with misfolding diseases. Here, we showed by thioflavin T fluorescence that the isomerization of L-Asp at different positions of amyloid β peptide 1–42 (Aβ42) generates opposing effects on its aggregation kinetics. We further determined the atomic structures of Aβ42 amyloid fibrils harboring a single D-Asp at position 23 and two D-Asp at positions 7 and 23 by cryo-electron microscopy helical reconstruction – cross-validated by cryo-electron tomography and atomic force microscopy – to reveal how D-Asp7 contributes to the formation of a unique triple stranded amyloid fibril structure stabilized by two threads of well-ordered water molecules. These findings provide crucial insights into how the conversion from L- to D-Asp influences the aggregation propensity and amyloid polymorphism of Aβ42.
Li-Ching Hsiao, Chih-Hsuan Lee, Karine Mazmanian, Masaya Yoshida, Genta Ito, Takuya Murata, Naoko Utsunomiya-Tate, Takeharu Haino, Shih-ichi Tate, Shang-Te Danny Hsu, Journal of Molecular Biology, 2025, 437, 169092
doi.org/10.1016/j.jmb.2025.169092
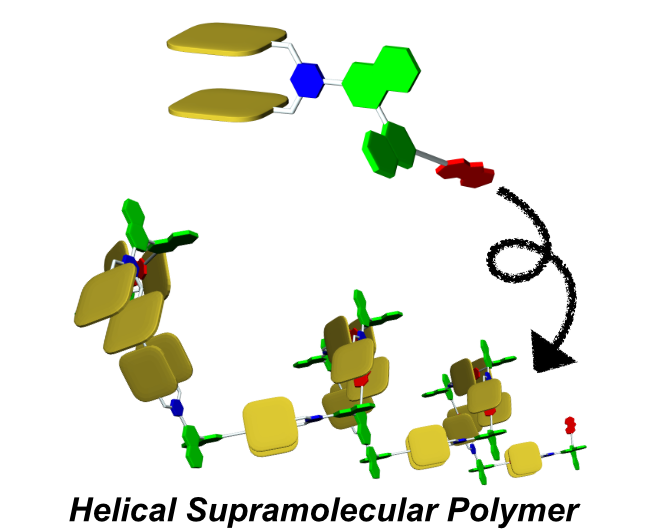
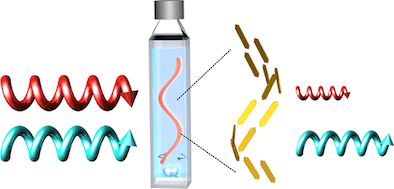
●Helical Supramolecular Polymers Formed via Head-to-Tail Host-Guest Complexation of Chiral Bisporphyrin Monomers with Trinitrofluorenone
The intermolecular host-guest complexation of head-to-tail monomers consisting of cleft-shaped bisporphyrin and trinitrofluorenone units connected by a chiral binaphthyl linker was employed to construct helically twisted supramolecular polymers. Results from 1H NMR, diffusion-ordered NMR spectroscopy, and viscometry experiments revealed that the supramolecular polymerization of these monomers follows a ring-chain competition mechanism. The introduction of bulky substituents at the linker significantly suppressed the formation of macrocyclic oligomers, whereas smaller alkyl chains facilitated the formation of the cyclic form. The chirally twisted structures of the supramolecular polymers were confirmed using circular dichroism spectroscopy. Atomic force microscopy revealed that the (R)- and (S)-configurations of the binaphthyl linkers induced right- and left-handed helical structures, respectively, in the supramolecular polymer chains. The absence of cooperativity in the supramolecular copolymerization of (R)- and (S)-1a resulted in the formation of stereo-random supramolecular copolymers.
Naoyuki Hisano, Tomoki Kodama, Soichiro Koya, Takeharu Haino, Chem. Eur. J., 2025, 31, e202404210.(Very Important Paper, Front Cover)
●Chirality Generation on Carbon Nanosheets by Chemical Modification
Chirality is an intriguing property of molecules, and an exciting area of study involves the generation of chirality in nanographene (NGs), also known as graphene quantum dots. Unlike those synthesized through stepwise carbon-carbon bond formation by organic reactions (bottom-up method), NGs are obtained by cutting parent carbons (top-down method) pose challenges in precisely regulating their three-dimensional structures by post-synthesis. This includes the incorporation of non-hexagonal rings and helicene-like structures in carbon frameworks. Currently, edge functionalization is the only method for generating chirality in NGs produced by the top-down method. While various chiral NGs have been synthesized through organic methods, examples of chemical modification remain rare due to limited structural information and the substantial size of NGs. However, these problems can be mitigated by disclosing the structures of NGs, particularly their edge structures. This minireview focuses on recently published papers that address the structural characterization of NGs and their chirality generation by edge modification. Comparing these NGs with those synthesized by organic synthesis will help to develop reasonable strategies for creating sophisticated chiral NGs. We hope this mini-review contributes to the advancement of NG-organic hybrid materials.
Ryo Sekiya, Saki Arimura, Haruka Moriguchi, Takeharu Haino, Nanoscale, 2025, 17, 774-787.
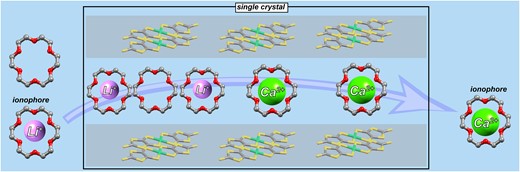
●Single-crystal-to-single-crystal transformation based on ionophore-like transport
We have already reported that Li2([18]crown-6)3[Ni(dmit)2]2(H2O)4 crystals, which have an ion channel structure, can be reversibly converted to Ca([18]crown-6)[Ni(dmit)2]2(H2O)3 crystals by ion exchange while maintaining their single-crystal state. In this process, inorganic ions as well as crown ethers traverse in and out of the crystal. In this study, [18]crown-6-d4, a partially deuterated [18]crown-6, was used to investigate the behavior of inorganic ions and crown ethers during ion exchange and found that Li+-[18]crown-6 supramolecules could act as ionophores in the crystal.
Mizuki Ito, Jun Manabe, Katsuya Inoue, Takehiro Hirao, Takeharu Haino, Tomoyuki Akutagawa, Kiyonori Takahashi, Takayoshi Nakamura, Sadafumi Nishihara, Chem. Lett., 54, 2025, upae252.
doi.org/10.1093/chemle/upae252
●Controlled Helical Organization in Supramolecular Polymers of Pseudo-Macrocyclic Tetrakisporphyrins
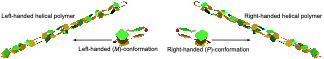
Tetrakisporphyrin monomers with amino acid side chains at each end form intramolecular antiparallel hydrogen-bonds to adopt chirally twisted pseudo-macrocyclic structures that result in right-handed and left-handed (P)- and (M)-conformations. The pseudo-macrocyclic tetrakisporphyrin monomers self-assembled to form supramolecular helical pseudo-polycatenane polymers via head-to-head complementary dimerization of the bisporphyrin cleft units in an isodesmic manner. The formation of one-handed supramolecular helical pseudo-polycatenane polymers was confirmed by circular dichroism spectroscopy. The methyl and iso-propyl groups at the stereogenic center greatly enhanced the induced circular dichroism (CD) in the Soret bands of the supramolecular helical pseudo-polycatenane polymers. The induced CDs were reduced upon the introduction of large iso-butyl and tert-butyl groups. Atomic force microscopy revealed well-grown and long supramolecular helical pseudo-polycatenane polymer chains with chain lengths in the range of 361 to 13.6 nm. The right-handed helical chains were established by the self-assembly of the right-handed (P)-conformation of the pseudo-macrocyclic monomer.
Naoka Fujii, Naoyuki Hisano, Takehiro Hirao, Shin-ichi Kihara, Kouta Tanabe, Masaya Yoshida, Shin-ichi Tate, Takeharu Haino, Angew. Chem. Int. Ed. 2025, 64, e202416770(Hot Paper, Inside Back Cover)
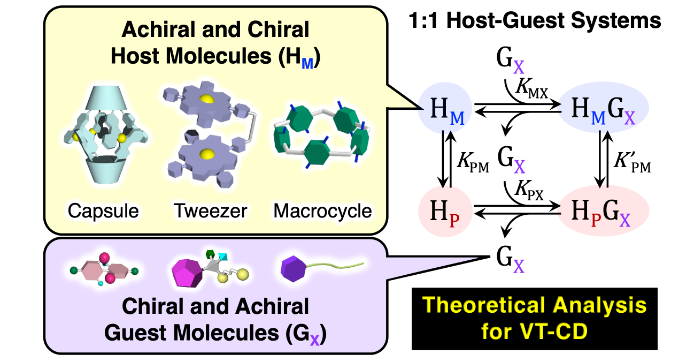
●Temperature-Dependent Left- and Right-Twisted Conformational Changes in 1:1 Host-Guest Systems: Theoretical Modeling and Chiroptical Simulations
An efficient strategy for high-performance chiral materials is to design and synthesize host molecules with left- and right- (M- and P-) twisted conformations and to control their twisted conformations. For this, a quantitative analysis is required to describe the chiroptical inversion, chiral transfer, and chiral recognition in the host-guest systems, which is generally performed using circular dichroism (CD) and/or proton nuclear magnetic resonance (1H-NMR) spectroscopies. However, the mass-balance model that considers the M- and P-twisted conformations has not yet been established. In this study, we derived the novel equations based on the mass-balance model for the 1:1 host-guest systems. Then, we further applied them to analyze the 1:1 host-guest systems for the achiral calixarene-based capsule molecule, achiral dimeric zinc porphyrin tweezer molecule, and chiral pillar[5]arene with the chiral and/or achiral guest molecules by using the data obtained from the CD titration, variable temperature CD (VT-CD), and 1H-NMR experiments. The thermodynamic parameters (ΔH and ΔS), equilibrium constants (K), and molar CD (Δε) in the 1:1 host-guest systems could be successfully determined by the theoretical analyses using the derived equations.
Nozomu Suzuki, Daisuke Taura, Yusuke Furuta, Yudai Ono, Senri Miyagi, Ryota Kameda, Takeharu Haino, Angew. Chem. Int. Ed. 2025, 64, e202413340.