Publication Lists
・Original Papers(2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002)
・Review&Books
2017

●Synthesis and Sturcture of Feet-to-Feet Connected Bisresorcinarenes

Bisresorcinarenes 1a–d were obtained in excellent yields, and 1e was finally obtained in 50% yield. X-ray diffraction analysis showed that 1a and 1b adopted helical conformations, whereas the two resorcinarenes of 1c–e were in parallel orientations in which the clefts of the aliphatic chains entrapped one or two solvent molecules. The conformational study revealed that the helix interconversion between the (P)- and (M)-helical conformers depended on the length of the aliphatic chains. 1a had the largest energetic barrier to helix interconversion, while in 1b, its more flexible aliphatic chains lowered its energetic barriers. The P/M interconversion of 1a was coupled with the clockwise/anticlockwise interconversion of the interannular hydrogen bonding of the two resorcinarenes. The large negative entropic contributions indicate that the transition state is most likely more ordered than the ground states, suggesting that the transition state is most likely symmetric and is solvated by water molecules. Calculations at the M06-2X/6-31G(d,p) level revealed that the more stable (P)-conformation has clockwise interannular hydrogen bonding between the two resorcinarenes.
Shimoyama, Daisuke; Ikeda, Toshiaki; Sekiya, Ryo; Haino, Takeharu, J. Org. Chem., 2017, 82, 13220-13230
●Hexameric Assembly of 5,17-di-Subsituted Calix[4]arene intheSolid State
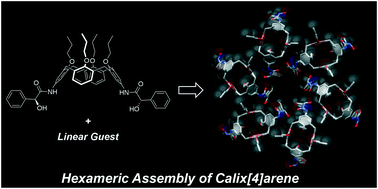
Chiral 5,17-difunctionalized-25,26,27,28-tetrapropyloxycalix[4]arene possessing (S)-mandelamide arms ((S,S)-1) afforded cocrystals (S,S)-1·(solvent) (solvent = MeOH, EtOH, 1-PrOH, 2-PrOH, and CH3CN). X-ray diffraction analysis revealed that (S,S)-1 formed head-to-tail columnar structures. In most cocrystals (MeOH, EtOH, 1-PrOH, and CH3CN), the columnar structures were arranged hexamerically to form a chiral hexagonal channel, which is a rare example of calix[4]arene derivatives in the solid state. The fact that the branched guest (2-PrOH) and the racemic mixture of the host formed different crystal packings wherein no hexameric structure existed indicates that both the linearity of the guest and the homochirality of the host are important factors for driving the columnar structures to form the hexameric assembly. Apohost (S,S)-1apo showed interesting adsorption behavior; it adsorbed benzene among the organic molecules tested. The adsorption and desorption processes were repeated several times, demonstrating the sponge-like character of (S,S)-1apo.
Yamasaki, Yutaro; Sekiya, Ryo; Haino, Takeharu, CrystEngComm, 2017, 19, 6744-6751(Front Cover)
●Slow IntermolecularComplexation-Decomplexation Exchanges of Cyclodextrins in Fullerene and Its DerivativeComplexes

Fullerenes (C60 and C70) and several functionalized C60 derivatives can be encapsulated in two γ‐cyclodextrins (γ‐CDxs). Although intermolecular complexation–decomplexation exchange of γ‐CDx is known to be very slow, the exchange rates have yet to be quantitatively measured. Herein, we determined that the pseudo‐first‐order association and dissociation rate constants for the γ‐CDx2•C70 complex were 4.3 and 0.6 s−1 at 23 °C, respectively. In contrast, the intermolecular exchange rates for the γ‐CDx2•C60 and C60 derivative complexes were slower than the time scale of exchange spectroscopy NMR experiments and the exchange rate constants were in the order of 10−4 s−1. Furthermore, a γ‐CDx2•C60 derivative complex was shown to have different intermolecular exchange rates for the two γ‐CDxs depending on steric hindrance from the substituent on the C60 derivative.
Ikeda, Atsushi; Mae, Tomoya; Sugikawa, Kouta; Komaguchi, Kenji; Konishi, Toshifumi; Hirao, Takehiro; Haino Takeharu, Chemistry Select, 2017, 2, 11322-11327.
●Sequence-Controlled Supramolecular Terpolymerization Directed by Specific Molecular Recognitions
Nature precisely manipulates primary monomer sequences in biopolymers.
In synthetic polymer sequences, this precision has been limited because
of the lack of polymerizationtechniques for conventional polymer synthesis.
Engineering the primary monomer sequence of a polymer main chain represents
a considerable challenge in polymer science. Here, we report the development
of sequence-controlled supramolecular terpolymerization via a self-sorting
behavior among three sets of monomers possessing mismatched host–guest
pairs. Complementary biscalix[5]arene-C60, bisporphyrin-trinitrofluorenone
(TNF), and Hamilton’s bis(acetamidopyridinyl)isophthalamide-barbiturate
hydrogen-bonding host–guest complexes are separately incorporated into
heteroditopic monomers that then generate an ABC sequence-controlled supramolecular
terpolymer. The polymeric nature of the supramolecular terpolymer is confirmed
in both solution and solid states. Our synthetic methodology may pave an
avenue for constructing polymers with tailored sequences that are associated
with advanced functions.
Hirao, Takehiro; Kudo, Hiroaki; Amimoto, Tomoko; Haino Takeharu, Nat. Commun.,2017,8, Article No. 634
DOI: 10.1038/s41467-017-00683-5
●Induced dipole-directed cooperative self-assembly of a benzotrithiophene

A benzotrithiophene derivative possessing phenylisoxazoles self-assembled to form stacks. The molecule isodesmically self-assembled in chloroform, whereas it self-assembled in a cooperative fashion in decalin and in methylcyclohexane. Thermodynamic studies based on isodesmic, van der Schoot, and Goldstein–Stryer mathematical models revealed that the self-assembly processes are enthalpically driven and entropically opposed. An enthalpy–entropy compensation plot indicates that the assembly processes in chloroform, decalin, and methylcyclohexane are closely related. The enthalpic gains in less-polar solvents are greater than those in more-polar solvents, resulting in the formation of large assemblies in decalin and in methylcyclohexane. The formation of large assemblies leads to cooperative assemblies. The elongation process is enthalpically more favored than the nucleation process, which drives the cooperativity of the self-assembly. DFT calculations suggested that a hexameric assembly is more stable than tetrameric or dimeric assemblies. Cooperative self-assemblies based on intermolecular interactions other than hydrogen bonding have rarely been reported. It is demonstrated herein that van der Waals interactions, including induced dipole–dipole interactions, can drive the cooperative assembly of planar π-conjugated molecules.
Ikeda, Toshiaki;Adachi, Hiroaki; Fueno, Hiroyuki; Tanaka, Kazuyoshi; Haino, Takeharu, J. Org. Chem., 2017, 82, 10062-10069.
●Supramolecular Polymeric Assemblies of pi - Conjugated Molecules Possessing Phenylisooxazoles
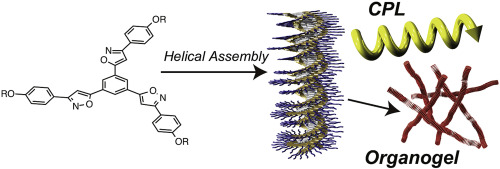
Supramolecular polymeric stacks of flat molecular components provide a highly ordered molecular organization that results in unique functions. The engineering of molecular organization, therefore, has attracted a great deal of attention both in supramolecular chemistry and in material science. The electronic and structural properties of molecular components determine the forthcoming supramolecular organization structure and properties. Non-covalent intermolecular interactions are responsible for directional growth in supramolecular polymerization. We have discovered that a phenylisoxazole ring system drives supramolecular polymerization in a directional way. π-Conjugated molecular components possessing phenylisoxazole moieties constructively assemble to form one-dimensional stacked assemblies via π–π stacking and dipole–dipole interaction. The introduction of a chirality onto the molecular components results in supramolecular helical organizations with unique photophysical functions. Switchable circularly polarized luminescence (CPL) and circular dichroism (CD) are developed in the supramolecular helical organizations. The grown assemblies provide highly entangled fibrillar networks, giving rise to organogels. The photo-responsive organogels and toroidal nanostructures are fabricated with the assistance of a photo-addressable azobenzene group. A light-harvesting system is developed in the organogels.
Ikeda, Toshiaki; Haino, Takeharu, Polymer,2017,128, 243-256
DOI: 10.1016/j.polymer.2017.02.05
●Photoluminescence Response of Graphene Quantom Dots toward Organic Bases and an Acid

Edge-modified graphene quantum dots (GQD-1) showed base-dependent photoluminescence (PL) responses. DBN, DBU, and Et3N gradually enhanced the PL intensity and then caused quenching, whereas quenching was not observed when pyridine and pyrimidine were used. The quenching can be explained by the photoinduced electron transfer from the anchored bases at the periphery to the emissive sites of GQD-1.
Suzuki, Kaho; Yamato, Kairi; Sekiya Ryo; Haino, Takeharu, Photochem. Photobio. Sci.2017,16, 623-626
●Supramolecular Graft Copolymerization of a Polyester via Guest-Selective Encapsulation of a Self-Assembled Capsule
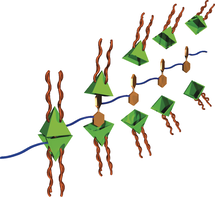
Repeating guest units of polyesters poly‐(R)‐2 were selectively encapsulated by capsule 1(BF4)4 to produce supramolecular graft polymers. The encapsulation of the guest units was confirmed by 1H NMR spectroscopy. The graft polymer structures were confirmed by the increase in the hydrodynamic radii and the solution viscosities of the polyesters upon complexation of the capsule. After the capsule was formed, atomic force microscopy showed extension of the polyester chains. The introduction of the graft chains onto poly‐(R)‐2 resulted in the main chain of the polymer having an M‐helical morphology. The complexation of copolymers poly‐[(R)‐2‐co‐(S)‐2] by the capsule gave rise to the unique chiral amplification known as the majority‐rules effect.
Tsunoda, Yuta; Takatsuka, Mei; Sekiya, Ryo; Haino Takeharu, Angew. Chem. Int. Ed.2017,56, 2613-2618
●Site-Selective Encapsulation of Different Anions in a Quadruply Interlocked Dimer
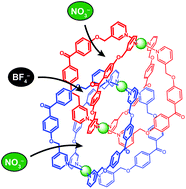
Interlocked dimer 2, which is composed of two physically interlocked monomers 1, has three cavities (cavity A × 2 and cavity B × 1) and can encapsulate three anions, such as NO3− and BF4−, one anion per cavity. There are six possible encapsulation patterns, A–F; two (A and F) contain only one kind of anion and the others (B–E) contain both NO3− and BF4− at the same time with different ratios and with different positions. Anion competition experiments showed that in addition to F, which encapsulates three NO3− ions, C, in which NO3− and BF4− ions are captured in cavities A and cavity B, respectively, was selectively formed. Detailed investigations have revealed that B–E were formed by dimerization, but three of the four were subjected to anion exchange and converged into C or F. This selective formation can be explained by the fact that NO3− is a better anion template than BF4−, as well as the molecular structure of the interlocked dimer; cavities A are surrounded by four bridging ligands and can be accessed by free anions, whereas no space available for anion exchange is present around cavity B because this cavity is surrounded by eight bridging ligands. Therefore, the BF4− ions in cavities A are expelled by free NO3−, but the BF4− ion in cavity B is not, resulting in the selection of C and F. We have found that the volume of the cavity influenced anion recognition. New interlocked dimer 3, which has smaller cavities than those of 2, captured three NO3− ions to form F, whereas only a small amount of an interlocked dimer that contains both NO3− and BF4− was formed.
Sekiya Ryo; Fukuda Morihiko; Kuroda Reiko, Org. Biomol. Chem.2017,15, 4328-4335
●Vanadium(V) Complexes of Some Bidentate Hydrazone Ligands and Their Bromoperoxidase Activity
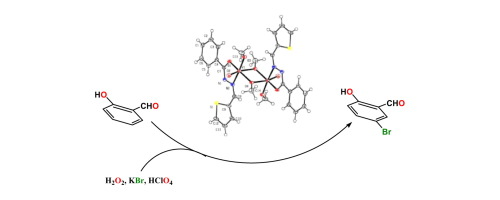
Dinuclear methoxy bridged complexes of vanadium, [VO(µ-OMe)(OMe)(L)]2 (1–3) have been synthesized from the reaction of VOSO4·H2O with triethylamine and the respective hydrazone ligand. The compounds have been characterized by spectroscopic methods and determination of single crystal X-ray structure of one of them (1). DFT and TD-DFT calculations were used to understand the electronic structures of the complexes and their electronic spectra respectively. Though the dimeric complexes are stable in the solid state, the ESI-MS spectra as well as 1H NMR spectra of the complexes suggest that in solution the monomeric forms of the complexes are the major species. The V(V) complexes in DMF were used to catalyze the oxidative bromination of salicylaldehyde, in aqueous H2O2/KBr in the presence of HClO4 at room temperature. The complexes show exceptionally high bromoperoxidase activity with salicylaldehyde as a model substrate to produce 5-bromo salicylaldehyde in good yield and high TOF and TON. Therefore, these complexes behave as functional models of vanadate dependent bromoperoxidase enzyme.
Adak, Piyali; Ghosh, Bipinbihari; Pakhira, Bholanath; Sekiya, Ryo; Kuroda, Reiko, Polyhedron,2017,127, 135-143
DOI:10.1016/j.poly.2017.01.054
●Light-harvesting organogel based on tris(phenylisoxazolyl)benzene
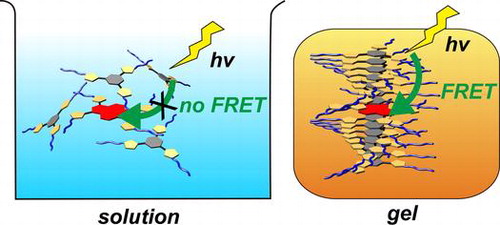
A novel light-harvesting organogel based on tris(phenylisoxazolyl)benzene, 1, was developed. A pyrene derivative, 2, possessing four phenylisoxazole substituents, was synthesised as an energy acceptor. The electronically excited-state energy of 1 was transferred to a small portion of 2 in the gel state, whereas the photo-induced energy transfer was not observed in solution. The coassembled structures of 1 and 2, formed in the decalin gel, exhibited light-harvesting behaviour. The study on the fluorescence quantum yield revealed that one molecule of 2 accepts the excited-state energy from approximately eight molecules of 1. Time-resolved fluorescence decay experiments revealed that the fluorescence resonance energy transfer is dominant in the energy transfer process in the gel state.
Ikeda, Toshiaki; Ueda, Yuko; Komori, Naomitsu; Abe, Manabu; Haino, Takeharu, Supramol. Chem., 2017, 29, 471-476
