Research
●Research Field of Our Group
Supramolecular chemistry focuses on self-assembled molecular architectures that are held together by supramolecular interactions, such as hydrogen bonding, van der Waals forces, and coulombic and solvophobic interactions. The association and dissociation of molecular constituents occurs simultaneously in a molecular assembly process. A subsequent self-assembled supramolecular architecture emerges as a thermodynamic minimum. Thus, supramolecular assembly offers quick access to structurally large and complex molecular architectures from the nano- to micrometer scales. Collaboration between supramolecular chemistry and material science has become one of the most effective bottom-up strategies to generate functional nano-size objects. Our group has been intensively working on the development of supramolecular assemblies and supramolecular polymers that are directed via unique molecular recognitions.

Prof. Takeharu Haino
●Supramolecular polymers based on molecular recognitions
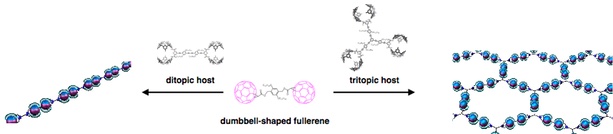
The formation of supramolecular polymers through host-guest complexation. The ditopic host (left) and the dumbbel-shaped fullerene gives one dimensional supramolecular polymers and the tritopic host (right) and the guest give two-dimensional supramolecular polymers.
A biscalix[5]arene–[60]fullerene supramolecular complex was utilized for the construction of supramolecular fullerene polymers. Di- and tritopic hosts were established to generate the linear and network supramolecular polymers via the molecular association of a dumbbell-shaped fullerene. The supramolecular fullerene polymers and networks were produced through intermolecular association of fullerene and the hosts. A mixture of the homoditopic host and thefullerenegave rise to fibers. Interdigitation of the alkyl side chains resulted in interchain interactions that enabled supramolecular organization. The homotritopic host produced the supramolecular networks with the dumbbell-shaped fullerene. Honeycomb architectures with a lot of voids were generated. The growth of the supramolecular polymers is obviously directed by the shape, dimension, and directionality of these monomers.
Representative articles
[1] Haino, T.; Matsumoto, Y.; Fukazawa, Y. J. Am. Chem. Soc. 2005, 127, 8936-8937.
[2] Hirao, T.; Tosaka, M.; Yamago, S.; Haino, T. Chem. Eur. J. 2014, 20, 16138-16146.
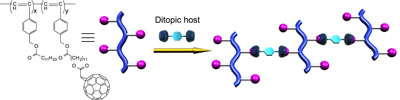
The formation of two-dimensional supramolecular polymers by mixing the ditopic hosts and the one-dimensional polymers carrying [60]fullerenes in their branches.
The reversibility of the cross-linkages can be given by using noncovalent bonds. Supramolecular entities were grafted onto conventional polymeric scaffolds to generate a cross-linked three-dimensional polymer network via noncovalent bonds. The homoditopic host selectively bound C60moieties grafted onto polyacetylene chains. This process resulted in a notably stable cross-linkage, increasing molecular weight and unique morphological transition to the polymer in its solid state. The stability of the supramolecular cross-linkage was directly associated with the solvent properties; therefore, the supramolecular cross-linkage of the polymer was determined by the solvent system. The macroscopic solid state morphologies of C60-grafted polyacetylenes were influenced by the supramolecular cross-linking. Nanoparticle-like morphologies were most likely prefered by the immiscible nature of the C60moiety. The supramolecular cross-linking produced the polymer bundle to give rise to uniform fibrils. These aligned into a well-oriented array on a HOPG surface.
Representative articles
[1] Haino, T.; Hirai, E.; Fujiwara, Y.; Kashihara, K. Angew. Chem. Int. Ed. 2010, 49, 7899–7903.
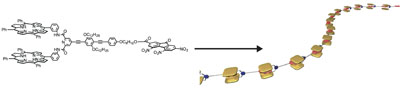
The formation of one-dimensional supramolecular polymers by self-assembly of monomers that carry a bisporphyrin donor and a fluorenone acceptor.
Supramolecular porphyrin polymers have attracted a great deal of attention because of their innovative applications in photoactive materials. A monomer, possessing a bisporphyrin cleft and a TNF moiety, assembled to form supramolecular polymers in solution and solid state. NMR studies evidenced that the polymer was produced via a head-to-tail host–guest complexation. The formation of entangled supramolecular polymers was evidenced by viscometry. Atomic force microscopy indicated that the porphyrin moiety settled on a perpendicular alignment that facilitated the oriented fibrillar nanoassemblies.
Representative articles
[1] Haino, T.; Watanabe, A.; Hirao, T.; Ikeda, T. Angew. Chem. Int. Ed. 2012, 51, 1473-1476.
[2] Kinjo, K.; Hirao, T.; Kihara, S.-i.; Katsumoto, Y.; Haino, T. Angew. Chem. Int. Ed. 2015, 54, in press.
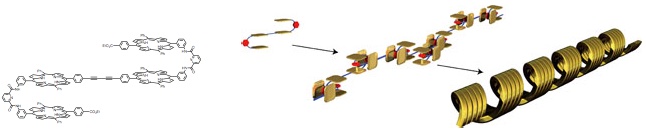
The formation of one dimensional supramolecular polymers by self-recognition of the ‘S’-shaped monomers. The polymers formed coiled morphologies on mica.
A S-shaped tetrakisporphyrin resulted in supramolecular polymeric assemblies directed by a complementary affinity of its bisporphyrin units. The self-association constant determined by the isodesmic model is larger than ten to the power of sixth L mol–1, suggesting that a sizable polymer forms at millimolar concentrations. The electron deficient aromatic guest bound within the molecular clefts given by the bisporphyrin units via a charge-transfer interaction. This guest complexation completely broke down supramolecular polymeric assembly. The long, fibrous fragments of the polymeric assemblies were determined by atomic force microscopy. The polymeric assemblies showed a coiled morphologies with a higher level of organization.
Representative articles
[1] Haino, T.; Fujii, T.; Watanabe, A.; Takayanagi, U. Proc. Natl. Acad. Sci. USA 2009, 106, 10477-10481.
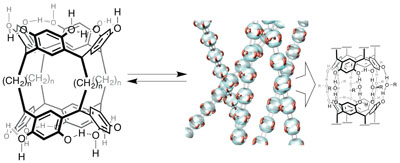
One-dimensional assemblies of bisresorcinarene.
The acid-catalyzed condensation of resorcinol and bisdimethoxyacetals resulted in rim-to-rim connected bisresorcinarenes in good yields. In the presence of ethanol, the homoditopic bisresorcinarenes assembled to generate supramolecular polymers with hydrogen bonding interactions. The fibrous morphologies of the supramolecular polymers were determined by microscopic methods. This bisresorcinarene should become a useful platform towards extensive preparation of resorcinarene-based host molecules.
The yield of the bisresorcinarenes are found to be improved considerably whenn-propanol is used as solvent and acyclic acetal is used as a regent. Some bisresorcinarenes can be obtained in nearly quantitative yield. The NMR analysis revealed that the bisresorcinarene with short alkyl chains (n= 8) undergoes interesting conformational interconversion. Clockwise conformation of the resorcinarene unit prefersP-helical structure of the four alkyl chains and vice versa. This indicates that the clockwise and counterclockwise interconversion leads to the helicity inversion of the four alky chains betweenP- andM-helical structures.
Representative articles
[1] Yamada, H.; Ikeda, T.; Mizuta, T.; Haino, T. Org. Lett. 2012, 14, 4510-4513.
[2] Shimoyama, D.; Ikeda, T.; Sekiya, R.; Haino, T. J. Org. Chem. 2017,82, 13220-13230.
●Supramolecular capsules constructed by calix[4]arenes
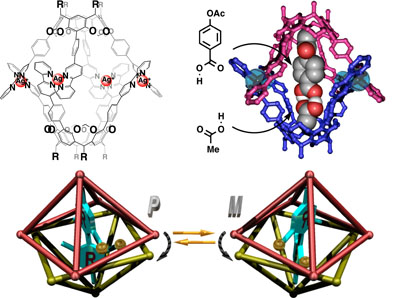
Supramolecular capsule composed of two resorcinarene cavitands. The capsule has P- or M-chirality. The chirality can be controlled by guest molecules.
Cavitand-based self-assembled coordination capsule provides unique binding space within the cavity. The cavity gave rise to a highly rigid binding space in which molecules with lengths of approximately 14 Å were selectively encapsulated. The rigid cavity recognized tiny structural differences in the flexible alkyl-chain guests as well as the rigid aromatic guests. The detailed thermodynamic studies indicated not only CH–π interactions between the methyl groups on the guest termini and the aromatic cavity walls, but also desolvation of the inner cavity play a important role in the guest encapsulation. The cavity found the hydrogen-bonded heterodimers of a mixture of two or three carboxylic acids as a complement.
Representative articles
[1] Haino, T.; Kobayashi, M.; Chikaraishi, M.; Fukazawa, Y. Chem. Commun. 2005, 2321-2323.
[2] Haino, T.; Kobayashi, M.; Fukazawa, Y. Chem. Eur. J. 2006, 12, 3310-3319.
[3] Haino, T.; Fukuta, K.; Iwamoto, H.; Iwata, S. Chem. Eur. J. 2009, 15, 13286-13290.
Encapsulation of chiral guests in the dissymmetric capsule resulted diastereomeric supramolecular complexes. When chiral guests were accommodated within the dissymmetric space of the self-assembled capsule, CD was observed at the absorption bands, characteristic of the π–π* transition of the bipyridine moiety of the capsule, indicating that theP- andM- helicities of the capsule are biased by the chiral guest complexation. The diastereo-selectivity was associated with the ester substituents, such that benzyl ester moieties were good for improving the diastereo-selectivity. A diastereomeric excess of 98 % was given. The relative enthalpic and entropic components for the diastereo-selectivity were given from a van’t Hoff plot. The enthalpic components were linearly correlated with the substituent Hammett parameters. The electron-rich benzyl ester moieties generated donor–acceptor stacking interactions with the bipyridine moiety, resulting in a large difference in energy between the predominant and subordinate diastereomeric complexes.
Representative articles
[1] Tsunoda, Y.; Fukuta, K.; Imamura, T.; Sekiya, R.; Furuyama, T.; Kobayashi, N.; Haino, T. Angew. Chem. Int. Ed. 2014, 53, 7243-7247.
●Helical stacks of planar π-conjugated molecules
Helical structure is ubiquitous in nature. DNA is a typical examples. Our group has been focused on the dynamic nature of supramolecular helical assemblies. Now, we are working on creating unique photochemical functions on supramolecular helical structures.
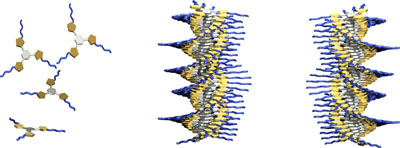
The formation of P- and M-helical assemblies of tris-isooxazoles.
Organogelators based on tris(isoxazolyl)benzene assemble through only weak dipole–dipole and π–π stacking interactions to create helical structures both in the gel and in solution, and the helical structures are influenced by an tiny proportion of the chiral monomer on the overall helical conformation.
Representative articles
[1] Haino, T.; Tanaka, M.; Fukazawa, Y. Chem. Commun. 2008, 468-470.
[2] Tanaka, M.; Ikeda, T.; Mack, J.; Kobayashi, N.; Haino, T. J. Org. Chem. 2011, 76, 5082-5091.
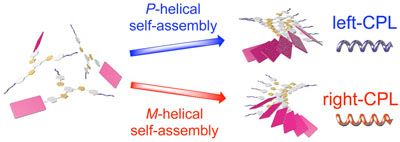
Circular polarized luminescence from one-dimensional supramolecular assemblies. The P-helical structure emits left-handed CPL and M-helical structure emits right-handed CPL.
The unique chiroptical properties induced by the chiral self-assembly of the tris(phenylisoxazolyl)benzenes possessing a PBI moiety. The self-assembling behaviors ofS- andR-tris(phenylisoxazolyl)benzenes were discussed. The chiral tris(phenylisoxazolyl)benzenes assembled to form the helical stacks in decalin. The CD signals were enhanced with decreasing temperature in decalin.S- andR-tris(phenylisoxazolyl)benzenes exhibited CPL with glum= 0.007 in decalin. The photophysical properties including UV–vis absorption, fluorescence, CD, and CPL are now controllable by changing the solvent, temperature, and concentration.
Representative articles
[1] Ikeda, T.; Masuda, T.; Hirao, T.; Yuasa, J.; Tsumatori, H.; Kawai, T.; Haino, T. Chem. Commun. 2012, 48, 6025-6027.
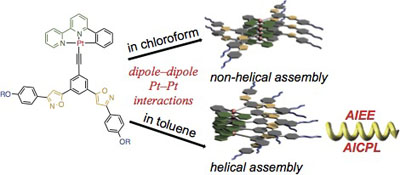
BIs(phenylisoxazolyl)phenylacetylene forms two different polymeric assemblies. In chloroform, they form non-helical assembly and the assembly does not show any aggregation-induced circularly polarized luminescence (AICPL), whereas in toluene, helical assemblies are formed and show AICPL.
Pt(II) phenylbipyridine complexes possessing bis(phenylisoxazolyl)phenylacetylene ligands assembled to give rise to stacked aggregates via Pt–Pt, π–π stacking, and dipole–dipole interactions. The assembled structures were determined by the solvent properties. Non-helical assemblies formed in chloroform displayed metal-metal-to-ligand charge transfer absorption and emission, while helical assemblies, produced in toluene, displayed aggregation-induced enhancement of emission and aggregation-induced circularly polarized luminescence. The rates of the association and dissociation of the assemblies were diminished in toluene, and the non-helical structures formed in chloroform were remarkably memorized.
Representative articles
[1] Ikeda, T.; Takayama, M.; Kumar, J.; Kawai, T.; Haino, T. Dalton Trans. 2015, 44, 13156-13162.
●Functional nanographenes

Nanographenes carring dendritic arms emit white-light upon excitation at 360 nm of light.
Nanographene, also known as graphene quantum dots, have received a great deal of attention for their potential applications in the development of novel optoelectronic devices. In the generation of optoelectronic devices, the development of nanographenes that are regulated in their size and dimensions and are un-oxidized at the sp2 surfaces is demanded. Nanographenes functionalized with bulky Fréchet’s dendritic wedges at the nanographene periphery were developed. The single-layered, size-regulated structures of the dendronized nanographenes were determined by atomic force microscopy. The edge-functionalization of the nanographenes resulted in white-light emission, which is an uncommon feature.
Representative articles
[1] Sekiya, R.; Uemura, Y.; Murakami, H.; Haino, T. Angew. Chem. Int. Ed. 2014, 53, 5619-5623.
●Molecular recognition in the solid state
Molecules without molecular recognition abilities in solution sometimes form co-crystals with other small organic molecules such as solvent molecules. These kinds of crystals are called as inclusion compounds or clathrates. In these crystals, small molecules (guests) are trapped in space between the molecules (hosts). Our group has interest in the molecular recognition in the solid state. In solution, the information on the interaction between molecules is indirectly obtained by nuclear magnetic resonance (NMR) spectroscopy, whereas in the solid state, the information can be directly and exactly (e.g., intermolecular distance) obtained by X-ray crystallography. This technique gives us a variety of the information on the interaction between host and guest, which is very useful for designing new host molecules.
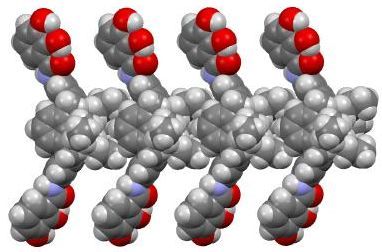
X-ray crystal structure of the one-dimensional polymeric array of calix[4]arene with two side arms.
Very recently, our group has found the formation of the polymeric one-dimensional array of calix[4]arene with two side arms in crystals. In these crystals, small organic molecules such as alcohol molecules were trapped in spaces formed between the polymeric arrays. We have also found the formation of the host-guest complexed between calix[5]arene (host) and linear alkane (guest) in the solid state. The host recognized the termini of the guests, and the termini of the guests formed unusual folded conformation. The formation of such energetically unfavorable conformations would be due to the maximization of the intermolecular contacts between the host and the guest. When the host formed co-crystals with monoalkylbenzenes, the calix[5]arene selectively included the alkyl chain. This finding suggests that the cavity of the calix[5]arene, which has a bowl-shaped cavity, prefers sp3 carbons to sp2 ones.
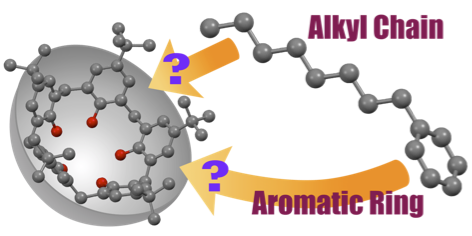
tert-Butylcalix[5]arene selectively recognizes the alphatic chains of monoalkylbenzenes in the solid state.
Representative articles
[1] Bull. Chem. Soc. Jpn. 2016, 89, 220-222.
[2] CrystEngComm. 2014, 16, 6023-6032.