Publication Lists
・Original Papers(2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002)
・Review&Books
2013


●Head-to-tail Polymeric Columnar Structure of Calix[4]arene Possessing Catechol Arms in the Solid State
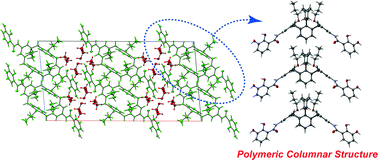
Calix[4]arene 1 cocrystallized with methanol (MeOH), ethanol (EtOH), isopropanol (iPrOH) and 1,4-butanediol (BDO) to afford clathrate compounds 1·(MeOH)4, 1·(EtOH)4, 1·(iPrOH)4, and 1·(BDO)2, respectively. The unique head-to-tail polymeric columnar structures of 1 were found in the solid state.
Sekiya, Ryo; Yamasaki, Yutaro; Katayama, Susumu; Shio, Hidemi; Haino, Takeharu, CrystEngComm, 2013, 15, 8404-8407
●Synthesis of Optically Active Poly(m-phenyleneethynylene-aryleneethynylene)s Bearing Hydroxy Groups and Examination of the Higher Order Structures
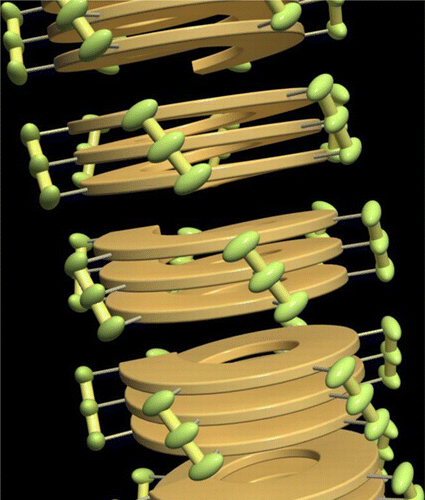
Novel optically active poly(m-phenyleneethynylene–aryleneethynylene)s bearing hydroxy groups with various arylene units {poly[(S)-/(R)-1–3a]–poly[(R)-1–3e] and poly[(S)-2–3a]} were synthesized by the Sonogashira–Hagihara coupling polymerization of 3,5-diiodo-4-hydroxy-C6H4CONHCH(CH3)COXC12H25 [(S)-/(R)-1 (X = O), (S)-2 (X = NH)] with HC≡C–Ar–C≡CH [3a (Ar = 1,4-C6H4), 3b (Ar = 1,4-C6H4-1,4-C6H4−), 3c (Ar = 1,4-C6H4-1,4-C6H4-1,4-C6H4−), 3d (Ar = 2,5-dihexyl-1,4-C6H2), 3e (Ar = 2,5-didodecyl-1,4-C6H2)]. The yields and number-average molecular weights of the polymers were in the ranges 60–94% and 7,000–29,500 with no correlation between the yield and the Mn. Circular dichroism (CD), UV–vis, and fluorescence spectroscopic analyses indicated that poly[(S)-1–3a]–poly[(S)-1–3c] and poly[(S)-2–3a] formed predominantly one-handed helical structures in THF, while poly[(S)-1–3d] and poly[(S)-1–3e] showed no evidence for forming chirally ordered structures. All polymers emitted blue fluorescence. The solution state IR measurement revealed the presence of intramolecular hydrogen bonding between the amide groups at the side chains of poly[(S)-1–2a]. The helical structures and helix-forming abilities of the polymers were analyzed by the molecular mechanics (MM), semiempirical molecular orbital (MO) and density functional theory (DFT) methods. Tube-like structures, presumably formed by perpendicular aggregation of the helical polymers, were observed by atomic force microscopy (AFM).
Sogawa, Hiromitsu; Shiotsuki, Masashi; Hirao, Takehiro; Haino, Takeharu; Sanda, Fumio, Macromolecules, 2013, 46, 8161-8170
●Size- and Orientation-Selective Encapsulation of C70by Cycloparaphenylenes
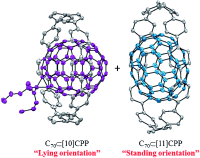
The size‐ and orientation‐selective formation of the shortest‐possible C70 peapod in solution and in the solid state by using the shortest structural unit of an “armchair” carbon nanotube (CNT), cycloparaphenylene (CPP), has been studied. [10]CPP and [11]CPP exothermically formed 1:1 complexes with C70, thereby giving the resulting peapods. A van′t Hoff plot analysis revealed that the formation of these complexes in 1,2‐dichlorobenzene was mainly driven by entropy, whereas the theoretical calculations suggested that the formation of the complex in the gas phase was predominantly driven by enthalpy. C70 was found to exist in two distinct orientations inside the CPP cavity, namely “lying” and “standing”, depending on the specific size of the CPP. The theoretical calculations and the X‐ray crystallographic analysis revealed that the interactions between [10]CPP and the short axis of C70 in its lying orientation were isotropic and similar to those observed between [10]CPP and C60. However, the interactions between [11]CPP and C70 in its standing orientation were anisotropic, thereby involving the radial deformation of [11]CPP into an ellipsoidal shape. This “induced fit” maximized the van der Waals interactions with the long axis of C70. Theoretical calculations revealed that the deformation occurred readily with low energy loss, thus suggesting that CPPs are highly radially elastic molecules. These results also indicate that the same type of radial deformation should occur in CNT peapods that encapsulate anisotropic fullerenes.
Iwamoto, Takahiro; Watanabe, Yoshiki; Takaya, Hikaru; Haino, Takeharu; Yasuda, Nobuhiro; Yamago, Shigeru, Chem. Eur. J., 2013, 19, 14061-1406(Cover Picture)
●Selective Synthesis of [2]- and [3]Catenane Tuned by Ring Size and Concentration
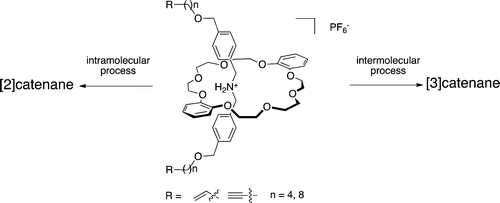
The syntheses of [2]- and [3]catenanes by olefin metathesis and oxidative acetylide coupling have been studied in detail. Pseudorotaxanes that were obtained by mixing crown ether and ammonium salts containing two terminal reactive end-groups were converted to [2]- and [3]catenane. Their yields were influenced not only by the chain length of the ammonium salts but also by the concentration of the crown ether and the ammonium salts. The strain energies of [2]catenane were responsible for the formation of [2]catenane.
Iwamoto, Hajime; Takizawa, Wataru; Itoh, Koji; Hagiwara, Tatsuya; Tayama, Eiji; Hasegawa, Eietsu; Haino, Takeharu, J. Org. Chem., 2013, 78, 5205-5217
●Photoresponsive Two-Component Organogelators based on Trisphenylisoxazolylbenzene
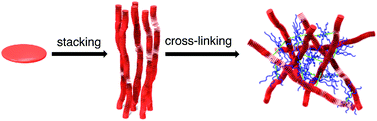
Photochromic tris(phenylisoxazolyl)benzene 1 and bispyridine derivatives 2a–e were mixed in a certain ratio to generate stable gels in benzyl alcohol, 4-methoxybenzyl alcohol, and aniline. Supramolecular assembly of 1 in solution was confirmed by 1H NMR study. The Tgel value was saturated in a 2 : 3 ratio of 1 and 2c. The intermolecular hydrogen bonds OH⋯N and salt bridge O−⋯H–N+ between 1 and 2c coexisted evidently, and these hydrogen bonds contributed to the stabilization of the gel networks. The lengths of alkyl chains of 2a–e governed the stabilities of the gels. The gel formations were driven by the morphological transition of 1 before and after the addition of 2a–e. Mixtures of 1 and 2a–e led to the well developed fibrillar networks, generating a lot of voids that are responsible for immobilizing solvent molecules. When the benzyl alcohol gel was irradiated at 360 nm, the gel turned to the sol. The sol was reversed to the gel by warming. This gel-to-sol phase transition was completely reversible.
Haino, Takeharu; Hirai, Yuko; Ikeda, Toshiaki; Saito, Hiroshi, Org. Biomol. Chem., 2013, 11, 4164-4170(Cover Picture)