Publication Lists
・Original Papers(2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002)
・Review&Books
2015


●Supramolecular Porphyrin Copolymer Assembled through Host-Guest Interactions and Metal-Ligand Coordination
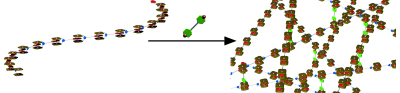
Bisporphyrin cleft molecule 1 Zn possessing a guest moiety assembled to form supramolecular polymers through host–guest interactions. Bispyridine cross‐linkers created interchain connections among the supramolecular polymers to form networked polymers in solution. Solution viscometry confirmed that the cross‐linked supramolecular polymers were highly entangled. Frequency‐dependent linear viscoelastic spectroscopy revealed that the supramolecular polymers generated well‐entangled solutions with associating and networking polymers, whereas the solid‐like aggregates moved individually without breaking and reforming structures below the transition temperature of 9.6 °C. Morphological transition of the supramolecular polymers was evidenced by AFM images; the non‐cross‐linked polymer resulted in wide‐spread thin networks, while the cross‐linked networks produced thicker worm‐like nanostructures. The supramolecular networks gelled in 1,1,2,2‐tetrachloroethane, and an elastic free‐standing film was fabricated with a Young’s modulus of 1 GPa.
Kinjo, Kanashi; Hirao, Takehiro; Kihara, Shin-ichi; Katsumoto, Yukiteru; Haino, Takeharu, Angew. Chem. Int. Ed., 2015, 54, 14830-14834
●Synthesis of Optically Active Conjugated Polymers Containing Platinum in the Main Chain: Control of the Higher-Order Structures by Substituents and Solvents
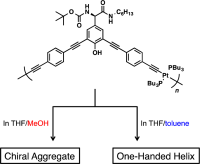
The Sonogashira–Hagihara coupling polymerization of d‐hydroxyphenylglycine‐derived diiodo monomers 1–4 and platinum‐containing diethynyl monomer 5 gave the corresponding polymers [poly(1–5)–(2–5)] with number‐average molecular weights of 19,000–25,000 quantitatively. The polymers were soluble in CHCl3, CH2Cl2, THF, and DMF. CD and UV–vis spectroscopic analysis revealed that amide‐substituted polymers [poly(1–5) and poly(2–5)] formed chiral higher‐order structures in solution, while ester‐substituted polymers [poly(3–5) and poly(4–5)] did not. Poly(1–5) formed one‐handed helices in THF/toluene mixtures, while it formed chiral aggregates in THF/MeOH mixtures. Poly(1–5) emitted fluorescence with quantum yields ranging from 0.8 to 1.3%. The polymers usually aggregated in the solid state.
Miyagi, Yu; Hirao, Takehiro; Haino, Takeharu; Sanda, Fumio, J. Polym. Sci. A Polym. Chem., 2015, 53, 2452-2461
●New Insights into Metal Ion-crown Ether Complexes Revealed by SEIRA Spectroscopy
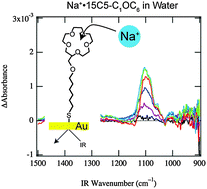
We demonstrate a powerful spectroscopic technique, surface-enhanced infrared absorption (SEIRA) spectroscopy, not only for detecting host–guest complexes in solution but also for examining the relationship between the guest selectivity, complex structure, and solvent effect. We synthesize thiol derivatives of 15-crown-5 and 18-crown-6 [2-(6-mercaptohexyloxy)methyl-15-crown-5 (15C5-C1OC6-SH) and 2-(6-mercaptohexyloxy)methyl-18-crown-6 (18C6-C1OC6-SH)], which are adsorbed on gold surfaces through S–Au bonds. The IR difference spectra of the M+·15C5-C1OC6 (M = Li, Na, K, Rb, and Cs) complexes on gold are observed using aqueous solutions of MCl by SEIRA spectroscopy. The spectra show a noticeable change in the C–O stretching vibration at around 1100 cm−1. The spectral patterns of M+·15C5-C1OC6 are similar for Li+ and Na+, and for K+, Rb+, and Cs+; the interaction between the metal ions and 15C5-C1OC6 changes drastically between Na+ and K+ in the series of alkali metal ions. On the other hand, the equilibrium constant for complex formation determined by the IR intensity shows clear preference for Na+ ions. We also observe the IR difference spectra of M+·18C6-C1OC6 in methanol and compare them with those in water. The spectral patterns in methanol are almost the same as those in water, but the equilibrium constant in methanol does not show preference for any ion, different from the K+ preference in water. From these findings we attribute the origin of the ion selectivity of 15C5 and 18C6 in solution to the interaction between the metal ions and the crown ethers in the complexes or the solvation energy of free ions. In the case of 15C5-C1OC6 in water, the preference of Na+ over K+, Rb+, and Cs+ can be attributed to the strength of the interaction or the size matching between metal ions and 15C5-C1OC6; the Na+ selectivity over Li+ ions is dominated by the solvation energy of free ions. For 18C6-C1OC6 in methanol, the equilibrium constant for complex formation becomes much bigger in methanol than that in water and loses the selectivity in methanol, because the solvation energy in methanol is fairly smaller than that in water, predominating the contribution from the strength of the interaction between metal ions and 18C6-C1OC6. The IR spectra measured by SEIRA spectroscopy are quite sensitive to properties of host–guest complexes such as the intermolecular interaction, the structure, and the orientation against the gold surface. However, the evidence for guest selectivity emerges primarily in the intensity of the spectra, rather than band positions or spectral patterns in the IR spectra.
Inokuchi, Yoshiya;Ebata, Takayuki; Ikeda, Toshiaki; Haino, Takeharu; Kimura, Tetsunari; Guo, Hao; Furutani, Yuji, New J. Chem., 2015, 39, 8673-8680
●UV Photodissociation Spectroscopy of Cryogenically Cooled Gas Phase Host-guest Complex Ions of Crown Ethers
The geometric and electronic structures of cold host–guest complex ions of crown ethers (CEs) in the gas phase have been investigated by ultraviolet (UV) fragmentation spectroscopy. As host CEs, we chose 15-crown-5 (15C5), 18-crown-6 (18C6), 24-crown-8 (24C8), and dibenzo-24-crown-8 (DB24C8), and as guests protonated-aniline (aniline·H+) and protonated-dibenzylamine (dBAM·H+) were chosen. The ions generated by an electrospray ionization (ESI) source were cooled in a quadrupole ion-trap (QIT) using a cryogenic cooler, and UV spectra were obtained by UV photodissociation (UVPD) spectroscopy. UV spectroscopy was complemented by quantum chemical calculations of the most probable complex structures. The UV spectrum of aniline·H+·CEs is very sensitive to the symmetry of CEs; aniline·H+·18C6 shows a sharp electronic spectrum similar to aniline·H+, while aniline·H+·15C5 shows a very broad structure with poor Franck–Condon factors. In addition, a remarkable cage effect in the fragmentation process after UV excitation was observed in both complex ions. In aniline·H+·CE complexes, the cage effect completely removed the dissociation channels of the aniline·H+ moiety. A large difference in the fragmentation yield between dBAM·H+·18C6 and dBAM·H+·24C8 was observed due to a large barrier for releasing dBAM·H+ from the axis of rotaxane in the latter complex.
Inokuchi, Yoshiya; Haino, Takeharu; Sekiya, Ryo; Morishima, Fumiya; Dedonder, Claude; Feraud, Geraldine; Jouvet, Christophe; Ebata, Takayuki, Phys. Chem. Chem. Phys., 2015, 17, 25925-25934
●Liposome Collapse Resulting from an Allosteric Interaction between 2,6-Dimethyl-beta-Cyclodextrins and Lipids
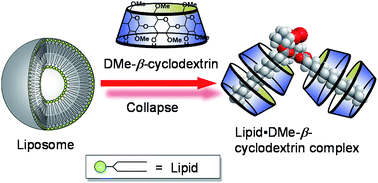
Although heptakis(2,6-di-O-methyl)-β-cyclodextrin (DMe-β-CDx) has been reported to exhibit higher cytotoxicity than many other cyclodextrins because of the way in which it abstracts cholesterols from liposomes, we have identified another reason for its cytotoxicity based on its interaction with lipids. These interactions exhibited nonlinear sigmoidal responses with Hill coefficient values (n) in the range of 3.0–3.6, which indicated that this phenomenon involves positive allosterism. Furthermore, analysis by mass spectroscopy revealed that the lipid–DMe-β-CDx complexes had stoichiometric ratios in the range of 1 : 1–1 : 4.
Ikeda, Atsushi; Iwata, Noboru; Hino, Shodai; Mae, Tomoya; Tsuchiya, Yuki; Sugikawa, Kouta; Hirao, Takehiro; Haino, Takeharu; Ohara, Kazuaki; Yamaguchi, Kentaro, RSC Adv., 2015, 5, 77746-77754
●Novel Helical Assembly of a Pt(II) Phenylbipyridine Complex Directed by Metal-metal Interaction and Aggregation-induced Circularly Polarized Emission

Pt(II) phenylbipyridine complexes possessing bis(phenylisoxazolyl)phenylacetylene ligands self-assembled to form stacked aggregates via Pt–Pt, π–π stacking, and dipole–dipole interactions. The assembled structures were influenced by the solvent properties. Non-helical assemblies found in chloroform displayed metal–metal-to-ligand charge transfer absorption and emission, whereas helical assemblies formed in toluene showed aggregation-induced enhancement of emission and aggregation-induced circularly polarized luminescence. The rates of the association and dissociation of the assemblies were significantly reduced in toluene, and the non-helical structures formed in chloroform were surprisingly memorized.
Ikeda, Toshiaki; Takayama, Midori; Kumar, Jatish; Kawai, Tsuyoshi; Haino, Takeharu, Dalton Trans., 2015, 44, 13156-13162
●Facile Construction of Well-defined Fullerene-dendrimer Supramolecular Nanocomposites for Bioapplications
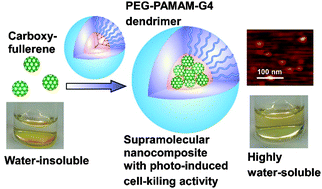
Well-defined fullerene–dendrimer supramolecular nanocomposites exhibiting uniform size, controlled morphology, high fullerene inclusion efficiency, excellent water solubility, and non-toxicity were facilely fabricated through complexation of carboxyfullerenes with poly(ethylene glycol)-modified poly(amidoamine) dendrimers.
Li, Xiaojie; Watanabe, Yasuo; Yuba, Eiji; Harada, Atsushi; Haino, Takeharu; Kono, Kenji, Chem. Commun., 2015, 51, 2851-2854
●Molecular Recognition of Upper Rim Functionalized Cavitand and Its Unique Dimeric Capsule in the Solid State
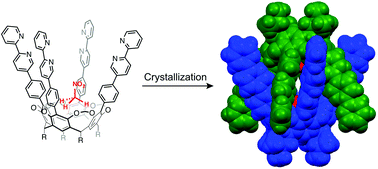
Cavitand 1 possesses four 2,2′-bipyridyl pillars on its upper rim that encapsulates small guests, such as nitromethane, acetonitrile, methyl acetate, ethyl acetate, and N-methylacetamide, into a deep cavity to form host–guest complexes in a 1 : 1 ratio. Nitroethane, N,N-dimethylformamide, and N,N-dimethylacetamide were not bound in this manner. A guest-binding study and molecular mechanics calculations revealed that the four 2,2′-bipyridyl pillars of cavitand 1 created a steric boundary that is responsible for selective guest recognition. In the solid state, cavitand 2 formed a unique chiral capsule 22 by π–π stacking interactions between the 2,2′-bipyridyl pillars. A nitromethane molecule wasunusually placed deep inside the cavity, as directed by the multiple hydrogen bonding interactions between the nitromethane oxygen atoms, the C–H bonds of the bridge methylenes and the pillar phenyl groups.
Kobayashi, Mutsumi; Takatsuka, Mei; Sekiya, Ryo; Haino, Takeharu, Org. Biomol. Chem., 2015, 13, 1647-1653(Cover Picture)
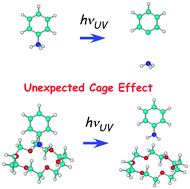
●Anomalous Cage Effect of the Excited State Dynamics of Catechol in the 18-Crown-6-Catechol Host-Guest Complex
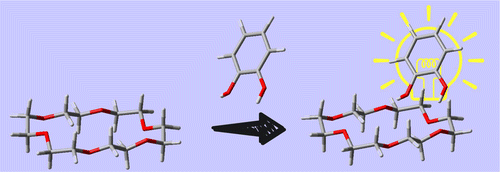
>We determined the number of isomers and their structures for the 18-crown-6 (18C6)–catechol host–guest complex, and examined the effect of the complex formation on the S1 (1ππ*) dynamics of catechol under a supersonically cooled gas phase condition and in cyclohexane solution at room temperature. In the gas phase experiment, UV–UV hole-burning spectra of the 18C6–catechol 1:1 complex indicate that there are three stable isomers. For bare catechol, it has been reported that two adjacent OH groups have an intramolecular hydrogen (H) bond. The IR–UV double resonance spectra show two types of isomers in the 18C6–catechol 1:1 complex; one of the three 18C6–catechol 1:1 isomers has the intramolecular H-bond between the two OH groups, while in the other two isomers the intramolecular H-bond is broken and the two OH groups are H-bonded to oxygen atoms of 18C6. The complex formation with 18C6 substantially elongates the S1 lifetime from 7 ps for bare catechol and 2.0 ns for the catechol–H2O complex to 10.3 ns for the 18C6–catechol 1:1 complex. Density functional theory calculations of the 18C6–catechol 1:1 complex suggest that this elongation is attributed to a larger energy gap between the S1 (1ππ*) and 1πσ* states than that of bare catechol or the catechol–H2O complex. In cyclohexane solution, the enhancement of the fluorescence intensity of catechol was found by adding 18C6, due to the formation of the 18C6–catechol complex in solution, and the complex has a longer S1 lifetime than that of catechol monomer. From the concentration dependence of the fluorescence intensity, we estimated the equilibrium constant K for the 18C6 + catechol ⇄ 18C6–catechol reaction. The obtained value (log K = 2.3) in cyclohexane is comparable to those for alkali metal ions or other molecular ions, indicating that 18C6 efficiently captures catechol in solution. Therefore, 18C6 can be used as a sensitive sensor of catechol derivatives in solution with its high ability of fluorescence enhancement.
Morishima, Fumiya; Kusaka, Ryoji; Haino, Takeharu; Ebata, Takayuk, J. Phys. Chem. B, 2015, 119, 2557-2565