Publication Lists
・Original Papers(2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002)
・Review&Books
2020







●Conformation of K+(Crown Ether) Complexes Revealed by Ion Mobility-Mass Spectrometry and Ultraviolet Spectroscopy

The conformation and electronic structure of dibenzo-24-crown-8 (DB24C8) complexes with K+ ion were examined by ion mobility–mass spectrometry (IM–MS), ultraviolet (UV) photodissociation (UVPD) spectroscopy in the gas phase, and fluorescence spectroscopy in solution. Three structural isomers of DB24C8 (SymDB24C8, Asym1DB24C8, and Asym2DB24C8) in which the relative positions of the two benzene rings were different from each other were investigated. The IM–MS results at 86 K revealed a clear separation of two sets of conformers for the K+(SymDB24C8) and K+(Asym1DB24C8) complexes whereas the K+(Asym2DB24C8) complex revealed only one set. The two sets of conformers were attributed to the open and closed forms in which the benzene–benzene distances in the complexes were long (>6 Å) and short (<6 Å), respectively. IM–MS at 300 K could not separate the two conformer sets of the K+(SymDB24C8) complex because the interconversion between the open and closed conformations occurred at 300 K and not at 86 K. The crown cavity of DB24C8 was wrapped around the K+ ion in the complex, although the IM–MS results availed direct evidence of rapid cavity deformation and the reconstruction of stable conformers at 300 K. The UVPD spectra of the K+(SymDB24C8) and K+(Asym1DB24C8) complexes at ∼10 K displayed broad features that were accompanied by a few sharp vibronic bands, which were attributable to the coexistence of multiple conformers. The fluorescence spectra obtained in a methanol solution suggested that the intramolecular excimer was formed only in K+(SymDB24C8) among the three complexes because only SymDB24C8 could possibly assume a parallel configuration between the two benzene rings upon K+ encapsulation. The encapsulation methods for K+ ion (the “wraparound” arrangement) are similar in the three structural isomers of DB24C8, although the difference in the relative positions of the two benzene rings affected the overall cross-section. This study demonstrated that temperature-controlled IM–MS coupled with the introduction of appropriate bulky groups, such as aromatic rings to host molecules, could reveal the dynamic aspects of encapsulation in host–guest systems.
Sota Tanaka, Yomoyuki Ujihira, Mayuko Kubo, Motoki Kida, Daisuke Shimoyama, Satoru Muramatsu, Manabu Abe, Takeharu Haino, Takayuki Ebata, Fuminori Misaizu, Keijiro Ohshimo, and Yoshiya Inokuchi, J. Phys. Chem. A, 2020, 124, 48, 9980–9990.
●Absorption of Chemicals in Amorphous Trisresorcinarene
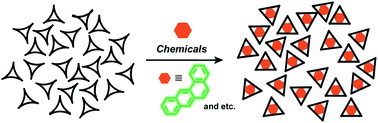
Trisresorcinarene is an interesting class of macrocyclic hosts. Its insoluble nature and conformationally flexible structure allow its application as a solid absorbent. Various aromatic and aliphatic hydrocarbons are absorbed into the amorphous solid of the trisresorcinarene, demonstrating the versatile absorption capability of trisresorcinarene.
Daisuke Shimoyama, Ryo Sekiya, and Takeharu Haino, Chem. Commun., 2020, 56, 12582-12585.
●One-Dimensional Arrangement of NORIA in the Solid-state
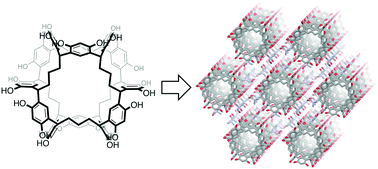
NORIA is a synthetic macrocycle consisting of twelve resorcinol rings. The hydroxy groups surrounding the surface of NORIA make it difficult for NORIA to be dissolved in organic solvents, except for polar solvents, a property which is ideal for developing solid-state materials. For developing solid-state materials, information about the effect of the crystallization conditions on the crystal packing of NORIA is required, although only limited examples have been reported so far. We find that the crystal packing of NORIA depends heavily on the crystallization conditions. Single crystals are grown in DMF solutions by the slow diffusion of pentane, cyclohexane, methylcyclohexane, benzene, and toluene into the solution. X-ray diffraction analysis demonstrates that the saturated hydrocarbon vapors induce the molecules to organize into a triclinic crystal packing, whereas another crystal packing, wherein NORIA is arranged linearly in the hexagonal crystal, is formed in the case of aromatic vapors. In the five crystals, DMF molecules are trapped by NORIA and interact with neighboring hosts through hydrogen bonding, indicating that the DMF molecules function as linkers. The X-ray crystal structures suggest that the planarity of the guests is a factor behind the formation of the hexagonal crystal packing. Electron density maps and the solvent accessible surface of NORIA suggest that small molecules, such as water, can access the cavity. The large solvent-accessible volumes in the unit cells of both crystal packings demonstrate that NORIA functions as an excellent building block for lattice inclusion compounds.
Daisuke Shimoyama, Ryo Sekiya, Hiroyuki Maekawa, Hiroto Kudo, and Takeharu Haino, CrystEngComm, 2020, 22 ,4740-4747
●A Dual Redox-Responsive Supramolecular Polymer Driven by Molecular Recognition between Bisporphyrin and Trinitrofluorenone
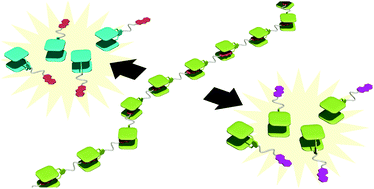
A dual redox-responsive supramolecular polymer driven by molecular recognition between bisporphyrin (bisPor) and trinitrofluorenone (TNF) has been developed. The supramolecular polymer was degraded into monomers in response to both oxidation and reduction stimuli.
Naoyuki Hisano, Takehiro Hirao, and Takeharu Haino, Chem. Commun., 2020 ,56 ,7553-7556(Inside Front Cover)
●Construction of Helically Stacked π‐Electron Systems in Poly(quinolylene‐2,3‐methylene) Stabilized by Intramolecular Hydrogen Bonds
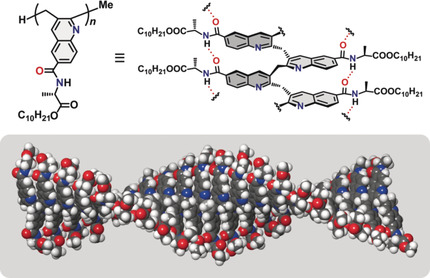
π‐Stacked polymers, which consist of layered π‐electron systems in a polymer, can be expected to be used in molecular electronic devices. However, the construction of a stable π‐stacked structure in a polymer is considerably challenging because it requires sophisticated designs and precise synthetic methods. Herein, we present a novel π‐stacked architecture based on poly(quinolylene‐2,3‐methylene) bearing alanine derivatives as the side chain, obtained through the living cyclo‐copolymerization of an o‐allenylaryl isocyanide. In the resulting polymer, the neighboring quinoline rings of the main chain form a layered structure with π–π interactions, which is stabilized by intramolecular hydrogen bonds. The vicinal quinoline units form two independent helices and the whole molecule is a twisted‐tape structure. This structure is established on the basis of UV/CD spectra, theoretical calculations, and atomic‐force microscopy.
Yuki Kataoka, Naoya Kanbayashi, Naoka Fujii, Taka-aki Okamura, Takeharu Haino, and Kiyotaka Onitsuka, Angew. Chem. Int. Ed .,2020 ,59 ,10286-10291
●Self‐Healing Supramolecular Materials Constructed by Copolymerization via Molecular Recognition of Cavitand‐Based Coordination Capsules
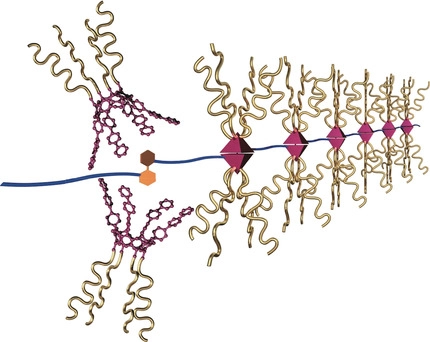
The repeating guest units of poly‐(R)‐2 were selectively encapsulated by the self‐assembled capsule poly‐1 possessing eight polymer side chains to form the supramolecular graft polymer (poly‐1 )n⋅poly‐(R)‐2 . The encapsulation of the guest units was confirmed by 1H NMR spectroscopy and the DOSY technique. The hydrodynamic radius of the graft polymer structure was greatly increased upon the complexation of poly‐1 . The supramolecular graft polymer (poly‐1 )n⋅poly‐(R)‐2 was stably formed in the 1:1 host–guest ratio, which increased the glass transition temperature by more than 10 °C compared to that of poly‐1 . AFM visualized that (poly‐1 )n⋅poly‐(R)‐2 formed the networked structure on mica. The (poly‐1 )n⋅poly‐(R)‐2 gelled in 1,1,2,2‐tetrachloroethane, which led to fabrication of distinct viscoelastic materials that demonstrated self‐healing behavior in a tensile test.
Natsumi Nitta, Mei Takatsuka, Shin-ichi Kihara, Takehiro Hirao, and Takeharu Haino, Angew. Chem. Int. Ed., 2020, 59, 16690-16697.
●Helicity of a polyacetylene directed by molecular recognition of biscalixarene and fullerene

Chiral biscalixarenes and a C60-appended polyphenylacetylene have been synthesized. The chiral biscalixarene encapsulated the C60 unit of the phenylpolyacetylene, which induced the preferred helicity of the polyacetylene.
Takehiro Hirao, Yoshiki Iwabe, Hisano Naoyuki, and Takeharu Haino, Chem. Commun., 2020, 56, 6672-6675
●Columnar Organization of Carbo[5]helicene Directed by Peripheral Steric Perturbation

Three carbo[5]helicenes ([5]CHs) containing benzylmaleimide groups displayed columnar stacked organizations, including dislocated, parallel, and alternating (P)- and (M)-helix molecular arrangements in the solid state. The peripheral benzyl groups resulted in very small steric interactions that are responsible for the formation of columnar stacked arrangements in the crystalline state.
Takehiro Hirao, Yudai Ono, Naomi Kawata, and Takeharu Haino, Org. Lett.,2020, 22, 5294-5298(Front Cover)
DOI:10.1021/acs.orglett.0c01421
●A Supramolecular Approach to Polymer-Shape Transformation via Calixarene-Fullerene Complexation

We synthesized fullerene-terminated polymethyl methacrylates (PMMAs), linear ditopic calix[5]arene host, and branched tritopic calix[5]arene host. The calix[5]arene hosts bound to the fullerene moieties of the PMMAs, inducing shape transformation among three different polymer shapes, namely, short PMMA, long PMMA, and star-shaped PMMA, in solution. The transformation was studied using UV/vis spectroscopy, fluorescence spectroscopy, diffusion ordered NMR spectroscopy, size-exclusion chromatography, viscometry, and differential scanning calorimetry. Dynamic light scattering measurements confirmed that the transformation between the individual shapes was induced by application of external stimuli, including prechosen molecules and heating of the solution. Of particular note is that atomic force microscopy revealed that PMMA illustrated an additional shape, namely, a spherical shape, in the solid state due to the cohesive nature of its fullerene moiety. The present study illustrated that a supramolecular approach to polymer-shape regulation allows access to multiple distinct polymer shapes in sequence.
Takehiro Hirao, Kazushi Fukuta, and Takeharu Haino, Macromolecules, 2020, 53, 3563–3570(Front Cover)
DOI:10.1021/acs.macromol.0c00621
●Upper-Rim Functionalization and Supramolecular Polymerization of a Feet-to-Feet-Connected Biscavitand

An octaiodobiscavitand was synthesized by an aromatic Finkelstein iodination reaction in good yield. Sonogashira and Suzuki coupling reactions of the octaiodobiscavitand gave rise to upper-rim-functionalized biscavitands that self-assembled to form the supramolecular polymer in the solid state.
Daisuke Shimoyama, Ryo Sekiya, and Takeharu Haino, Chem. Commun., 2020, 56, 3733-3736 .
●Folding control of a non-natural glycopeptide using saccharide-coded structural information for polypeptides

We synthesized “glyco-arylopeptides”, whose folding structure significantly changes depending on the kind of saccharide in their side chain. The saccharide moiety interacts with the main chain via hydrogen bonding, and the non-natural polypeptides form two well-defined architectures—(P)-31- and (M)-41-helices—depending on the length of the saccharide chains and even the configuration of a single stereo-genic center in the epimers.
Yuki Ishido, Naoya Kanbayashi, Naoka Fujii, Taka-aki Okayamura, Takeharu Haino, and Kiyotaka Onitsuka, Chem. Commun., 2020, 56, 2767-2770.
●A Regulable Internal Cavity Inside A Resorcinarene‐Based Hemi‐Carcerand

Covalent organic capsules, such as carcerands and hemi‐carcerands, constitute an interesting class of molecular hosts. These container molecules have confined spaces capable of hosting small molecules, although the size of the inner cavities cannot be changed substantially, limiting the scope of the applications. The title covalently linked container is produced by metal‐directed dimerization of a resorcinarene‐based cavitand possessing four 2,2’‐bipyridyl arms on the wide rim followed by olefin metathesis at the vertexes of the resulting capsule with a second‐generation Grubbs catalyst. The covalently linked bipyridyl arms permit expansion of the inner cavity by demetalation. This structural change influences the molecular recognition properties; the metal‐coordinated capsule recognizes only 4,4’‐diacetoxybiphenyl, while a metal‐free counterpart can encapsulate not only 4,4’‐diacetoxybiphenyl but also 2,5‐disubstituted‐1,4‐bis(4‐acetoxyphenylethynyl)benzene, which is 9.4 Å longer than the former guest. Molecular mechanics calculations predict that the capsule expands the internal cavity to encapsulate the long guest by unfolding the folded conformation of the alkyl chains, demonstrating the flexible and regulable nature of the cavity. The guest competition experiments show that the preferred guest can be switched by metalation and demetalation.
Kentaro Harada,. Ryo Sekiya, and Takeharu Haino, Chem. Eur. J., 2020, 26, 5810-5817(Front Cover)
●AIE-active Micells Formed by Self-assembly of an Amphiphilic Platinum Complex Possessing Isoxazole Moieties

An increased interest in the use of platinum complex based luminescent micelles for imaging biological tissues has emerged in recent years due to their low cytotoxicity, synthetic flexibility, photostability, and high emission efficiency. Here, we report a luminescent micelle that is prepared through the self-assembly of an amphiphilic, neutral Pt(II) complex with isoxazole moieties in THF/water on account of its aggregation-induced emission (AIE) property.
Takehiro Hirao, Hidemi Tsukamoto, Toshiaki Ikeda, and Takeharu Haino, Chem. Commun., 2020, 56, 1137-1140.
●Entropy–driven Cooperativity in the Guest Binding of an Octaphosphonate Biscavitand

Uncommon entropy‐driven cooperativity is reported in the guest binding of an octaphosphonate biscavitand. Isothermal titration calorimetry analysis determined the thermodynamic parameters for the 1:2 host‐guest binding of biscavitands with ammonium guests in methanol, ethanol, isopropanol, and chloroform. Chloroform drove uncommon entropy‐driven cooperative binding, whereas the alcohols resulted in enthalpy‐driven noncooperative binding. 1H NMR studies revealed that each cavity possessed six water molecules in chloroform, which were liberated upon the guest binding. The enthalpy‐entropy compensation relationship produced a large positive intrinsic entropy in chloroform, which implies that water desolvation causes a considerable entropic gain by paying an enthalpic penalty due to breaking the hydrogen bonding networks of the water clusters.
Daisuke Shimoyama and Takeharu Haino, Chem. Eur. J., 2020, 26, 3074-3079(Front Cover)
●Chirality-Embedded Nanographene

The development of chiral nanographenes hasmostly been carried out by bottom-up methods and examplesof species developed by the post-modification of nanogra-phenes prepared by top-down methods remain limited. Weshow that the attachment of chiral functional groups onto theedge of nanographenes generates chirality on the surface. X-ray diffraction analysis and DFT calculations indicate that thechirality of the functional groups is transferred to the surfacevia steric interactions from the chiral center through the five-membered cyclic imide to the nanographene edge. The excitoncoupling between the p-bromophenyl groups confirms that thefunctional groups are arranged on the armchair edges atdistances that permit exciton coupling, which provides infor-mation about their relative orientation. These pieces ofinformation help to elucidate the edge structure of nano-graphenes prepared by top-down methods.
Shohei Nishitani, Ryo Sekiya, and Takeharu Haino, Angew. Chem. Int. Ed., 2020, 59, 669-673(Hot Paper)
●Feet-to-feet Connected Trisresorcinarenes

The macrocyclization of resorcinol and odd-numbered bisdioxolanes under acidic conditions produced feet-to-feet connected trisresorcinarenes possessing three resorcinarene units linked with odd-numbered alkyl chains. The formation of trisresorcinarenes was confirmed using high-resolution mass spectrometry and NMR spectroscopy. The trisresorcinarenes were isolated as protected forms in moderate yields. The protected trisresorcinarenes exhibited D3h symmetry in conformation in solution. Crystal structure analysis revealed that the protected trisresorcinarenes possess large inner spaces surrounded by three resorcinarene units.
Daisuke Shimoyama, Ryo Sekiya, Hiroto Kudo, and Takeharu Haino, Org. Lett., 2020, 22, 352-356(Front Cover)