Publication Lists
・Original Papers(2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002)
・Review&Books
2018


●Facile Synthesis of an Eight-Armed Star-Shaped Polymer via Coordination-Driven Self-Assembly of a Four-Armed Cavitand

The polystyrene chains were installed at the lower rim of a resorcinarene-based cavitand via reversible addition–fragmentation (RAFT) polymerization to form a four-armed star-shaped polymer. A star-shaped polystyrene-functionalized supramolecular capsule was prepared through the coordination-driven self-assembly of the four-armed start-shaped polymer with silver ions. The eight-armed start-shaped supramolecular capsule encapsulated 4,4′-diacetoxybiphenyl as did a cavitand-based self-assembled capsule. A DOSY measurement indicated that the eight-armed star-shaped polymer was twice as large as the four-armed star-shaped polymer. The solution behaviors of these compounds resulted in a difference in their zero-shear viscosities.
Nitta, Natsumi; Mei, Takatsuka; Kihara, Shin-ichi; Sekiya, Ryo; Haino, Takeharu, ACS Macro. Lett., 2018, 7, 1308-1311(Front Cover)
DOI:10.1021/acsmacrolett.8b00669
●Majority-Rules Effect and Allostery in Molecular Recognition of Calix[4]arene-Based Triple-Stranded Metallohelicates

The triple‐stranded metallohelicates 1a,/1b–3a/3b possessing internal guest binding cavities surrounded by calix[4]arene units were synthesized through coordination‐driven self‐assembly. UV/Vis titration experiments verified that the metallohelicates encapsulated N‐methyl pyridinium cations bearing amino acid groups to form host–guest complexes. The guest chirality was transferred to the helicity of the helicates through the steric contact between the stereogenic center of the amino acid group and the metal cores. The (M) helicity was induced when guests the (R)‐4‐‐(R)‐6 were accommodated within the cavities. The multiple guest complexation within the self‐assembled helicates 2a and 3a displayed large positive cooperative effects, indicating that the first guest complexation preorganizes the rest of the cavities to facilitate a subsequent guest binding. This cooperativity results in the majority‐rules effect in the chiral guest binding for 2a and 3a.
Yamasaki, Yutaro; Shio, Hidemi; Amimoto, Tomoko; Sekiya, Ryo; Haino, Takeharu, Chem. Eur. J., 2018, 24, 8558-8568
●Supramolecular Properties of a Monocarboxylic Acid-Functionalized “Texas-Sized” Molecular Box

A new carboxylic acid-functionalized “Texas-sized” molecular box TxSB-CO2H has been prepared by combining two separate building blocks via an iodide-catalyzed macrocyclization reaction. A single-crystal X-ray diffraction analysis revealed a paired “clip-like” dimer in the solid state. Concentration-dependent behavior is seen for samples of TxSB-CO2H as prepared, as inferred from 1H NMR spectroscopic studies carried out in DMSO-d6. However, in the presence of excess acid (1% by weight of deuterated trifluoracetic acid; TFA-d1), little evidence of aggregation is seen in DMSO-d6 except at the highest accessible concentrations. In contrast, the conjugate base form, TxSB-CO2–, produced in situ via the addition of excess triethylamine to DMSO-d6 solutions of TxSB-CO2H acts as a self-complementary monomer that undergoes self-assembly to stabilize a formal oligomer ([TxSB-CO2–]n) with a degree of polymerization of approximately 5–6 at a concentration of 70 mM. Evidence in support of the proposed oligomerization of TxSB-CO2– in solution and in the solid state came from one- and two-dimensional 1H NMR spectroscopy, X-ray crystallography, dynamic light scattering (DLS), and scanning electron microscopy (SEM). A series of solution-based analyses carried out in DMSO and DMSO-d6 provide support for the notion that the self-assembled constructs produced from TxSB-CO2– are responsive to environmental stimuli, including exposure to the acetate anion (as its tetrabutylammonium, TBA+, salt), and changes in overall concentration, temperature, and protonation state. The resulting transformations are thought to reflect the reversible nature of the underlying noncovalent interactions. They also permit the stepwise interconversion between TxSB-CO2H and [TxSB-CO2–]n via the sequential addition of triethylamine and TFA-d1. The present work thus serves to illustrate how appropriately functionalized molecular box-type macrocycles may be used to develop versatile stimuli-responsive materials. It also highlights how aggregated forms seen in the solid state are not necessarily retained under competitive solution-phase conditions.
Wu, Ren-Tsung;Chi, Xiaodongi; Hirao, Takehiro;Lynch, Vincent M. Lynch; Sessler, Jonathan L, J. Am. Chem. Soc., 2018, 140, 6823-6831.
●Control over Multiple Molecular States with Directional Changes Driven by Molecular Recognition

Recently, ligand–metal coordination, stimuli-responsive covalent bonds, and mechanically interlinked molecular constructs have been used to create systems with a large number of accessible structural states. However, accessing a multiplicity of states in sequence from more than one direction and doing so without the need for external energetic inputs remain as unmet challenges, as does the use of relatively weak noncovalent interactions to stabilize the underlying forms. Here we report a system based on a bispyridine-substituted calix[4]pyrrole that allows access to six different discrete states with directional control via the combined use of metal-based self-assembly and molecular recognition. Switching can be induced by the selective addition or removal of appropriately chosen ionic guests. No light or redox changes are required. The tunable nature of the system has been established through a combination of spectroscopic techniques and single crystal X-ray diffraction analyses. The findings illustrate a new approach to creating information-rich functional materials.
Hirao, Takehiro; Kim, Dong Sub; Chi, Xiaodong;Lynch Vincent M.;Ohara, Kazuaki; Park, Jung Su; Yamaguchi, Kentaro; Sessler, Jonathan L., Nature Commun.,2018,9, 823
DOI:10.1038/s41467-018-03220-0
●Supramolecular Copolymerization by Sequence Reorganization of a Supramolecular Homopolymer

The homopolymeric sequence formed by the head‐to‐head association of tetrakisporphyrin 1 is completely dissociated by the competitive association of the ditopic guest G2, resulting in the supramolecular copolymer poly‐1⋅G2 with an alternatingly repeating host–guest sequence. The 1:1 stoichiometry of 1 and G2 is confirmed by a Job plot using UV/Vis titration and diffusion‐ordered NMR spectroscopy (DOSY). The solution viscometry for poly‐1 and poly‐1⋅G2 suggests that the supramolecular chain of poly‐1 behaves like a rod, whereas the supramolecular copolymer chain of poly‐1⋅G2 behaves like a swelled fat chain, which is entangled in the semi‐dilute regime. Atomic force microscopy shows that the supramolecular polymer poly‐1⋅G2 is highly oriented through the interdigitation of the long alkyl chains.
Nadamoto, Kouhei; Maruyama, Kei; Fujii, Naoka; Ikeda, Toshiaki; Kihara, shin-ichi; Haino, Takeharu, Angew. Chem. Int. Ed.,2018,57, 7028-7033
●Controllable Direction of Porphyrin Derivatives in Two Cyclodextrin Cavities

Porphyrin–trimethyl‐β‐cyclodextrin (TMe‐β‐CDx) complexes have pseudorotaxane structures in which two meso‐phenyl and/or ‐pyridyl moieties penetrate the upper rim of two TMe‐β‐CDx molecules. Porphyrin derivatives with one to three pyridyl moieties at meso positions formed complexes with TMe‐β‐CDx in which the penetration of the upper rim of the two TMe‐β‐CDxs by the pyridyl moieties was minimized. In contrast, in TMe‐β‐CDx complexes formed with porphyrin derivatives bearing two 2‐methoxyphenyl moieties and two pyridyl moieties, the pyridyl moieties penetrate the upper rim of the two molecules because steric hindrance prevents penetration by the 2‐methoxyphenyl moieties.
Horiguchi, Banri; Nakaya, Toshimi; Ueda, Masafumi; Sugisawa, Kouta; Mizuta, Tsutomu; Haino, Takeharu; Kawata, Naomi; Ikeda, Atsushi, Eur. J. Org. Chem.,2018,18, 2138-2143
●Pseudorotaxanes in the Gas Phase: Structure and Energetics of Protonated Dibenzylamine-crown Ether Complexes

We observe UV spectra of protonated dibenzylamine (dBAMH+) and its complexes with 15-crown-5 (dBAMH+–15C5), 18-crown-6 (dBAMH+–18C6), and 24-crown-8 (dBAMH+–24C8) under cold (∼10 K) gas-phase conditions by UV photodissociation (UVPD) and UV–UV hole-burning (HB) spectroscopy. The UVPD spectrum of the dBAMH+–15C5 complex shows an extensive low-frequency progression, which originates from a unique conformation of the dBAMH+ part with benzene rings facing closely to each other, while UVPD and calculation results suggest open conformations of the dBAMH+ part for dBAMH+–18C6 and dBAMH+–24C8. UV–UV HB spectra of the dBAMH+–24C8 complex indicate that there exist at least two conformers; multiple conformations can contribute to high stability of dBAMH+–24C8 pseudorotaxane due to “conformational” entropic effects. The UVPD experiment indicates that the dissociation probability of dBAMH+–24C8 into dBAMH+ and 24C8 is substantially smaller than that of dBAMH+–15C5 and dBAMH+–18C6, which can be related to the barrier height in the dissociation process. The energetics of the dBAMH+–24C8 complex is investigated experimentally with NMR spectroscopy and theoretically with the global reaction route mapping (GRRM) method. An energy barrier of ∼60 kJ mol−1 is present in the pseudorotaxane formation in solution, whereas there is no barrier in the gas phase. In the course of the photodissociation, excited dBAMH+–24C8 complexes can be trapped at many local minima corresponding to multiple conformations. This can result in effective dissipation of internal energy into degrees of freedom not correlated to the dissociation and decrease the dissociation probability for the dBAMH+–24C8 complex in the gas phase. The energy barrier for the pseudorotaxane formation in solution originates not simply from the slippage process but rather from solvent effects on the dBAMH+–24C8 complex.
Kida, Motoki; Shimoyama, Daisuke; Ikeda, Toshiki; Sekiya, Ryo; Haino, Takeharu; Ebata, Takayuki; Jouvet, Christophe; Inokuchi, Yoshiya, Phys. Chem. Chem. Phys.,2018,20, 18678-18687.
'
●A Supramolecular Polymer Network of Graphene Quantum Dots
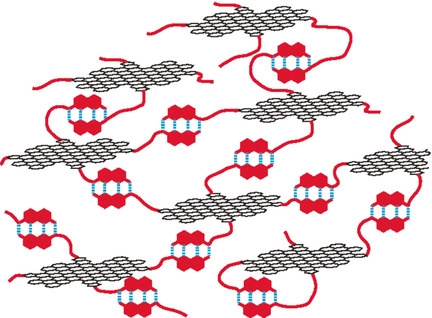
Graphene quantum dot (GQD)–organic hybrid compounds (GQD‐2b–e) were prepared by introducing 3,4,5‐tri(hexadecyloxy)benzyl groups (C16) and linear chains terminated with a 2‐ureido‐4‐[1H]‐pyrimidinone (UPy) moiety onto the periphery of GQD‐1. GQD‐2b–e formed supramolecular assemblies through hydrogen bonding between the UPy units. GPC analysis showed that GQDs with high loadings of the UPy group formed larger assemblies, and this trend was confirmed by DOSY and viscosity measurements. AFM images showed the polymeric network structures of GQD‐2e on mica with flat structures (ca. 1.1 nm in height), but no such structures were observed in GQD‐2a, which only carries the C16 group. GQD‐2c and GQD‐2d formed organogels in n‐decanol, and the gelation properties can be altered by replacing the alkyl chains in the UPy group with ethylene glycol chains (GQD‐3). GQD can thus be used as a platform for supramolecular polymers and organogelators by suitable chemical functionalization.
Uemura, Yuichiro; Yamato Kairi; Sekiya, Ryo; Haino, Takeharu, Angew. Chem. Int. Ed.,2018,57, 4960-4964
●Synthesis and Dimerization Studies of a Lipophilic Photoresponsive Aryl Extended Tetraureacalix[4]pyrrole
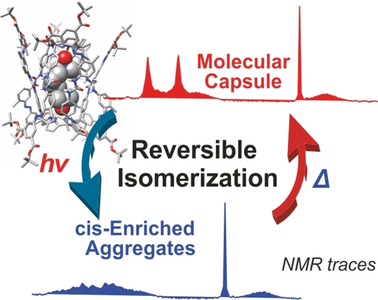
We describe the syntheses of the lipophilic aryl‐extended α,α,α,α‐tetraurea‐phenyl‐calix[4]pyrrole 1, featuring four appended azo‐phenyl groups with two tert‐butoxy carbonyl meta‐substituents and its photo‐inactive counterpart 2. In CD2Cl2 solutions, both tetraurea‐calix[4]pyrroles self‐assemble into dimeric capsules by encapsulating one molecule of a suitable bis‐N‐oxide or two molecules of a mono‐N‐oxide. The dimeric capsules are mainly stabilized by a cyclic array of sixteen hydrogen bonds established between the eight unidirectionally oriented urea groups. Photoirradiation experiments demonstrated the trans‐to‐cis isomerization of the azo‐phenyl groups and the formation of a plethora of stereo isomeric cis‐azo‐enriched capsular assemblies. The highly cis‐azo enriched capsular assemblies seem to show a reduced stability and their involvement in equilibria with non‐capsular counterparts that also bind the N‐oxides. The thermally induced cis‐to‐trans interconversion processes demonstrated the reversibility of the photoisomerization and the photostability of most binding partners. An equimolar mixture of the two tetraureas produced two homodimeric capsules and the heterodimeric counterpart in a ratio close to statistical distribution.
Sekiya, Ryo; Díaz-Moscoso, Alejandro; Ballester, Pablo, Chem. -Eur. J., 2018, 24, 2182-2191
●A Circularly Polarized Luminescent Organogel Based on a Pt(II) Complex Possessing Phenylisoxazoles
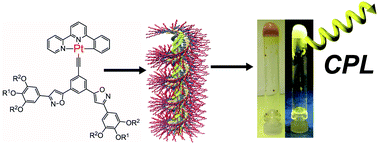
A Pt(II)phenylbipyridine complex 1, possessing phenylisoxazoles, long alkyl chains, and chiral alkyl chains, was synthesized. Complex 1 formed a stacked assembly in chloroform, self-assembled in a cooperative manner in methylcyclohexane (MCH), and gelled in higher alcohols and dodecane. In the nucleation regime, 1 assembled via a Pt–Pt interaction, which was demonstrated by the metal–metal-to-ligand charge transfer (MMLCT) emission band of the assembly. In the elongation regime, 1 displayed aggregation-induced emission enhancement (AIEE) character. The assembly of 1 formed in MCH was chiroptically non-active, suggesting that the assembly was non-helical. Complex 1 also exhibited strong AIEE and circularly polarized luminescence (CPL) with an anisotropic factor (glum) of 0.011 in 1-decanol gel, indicating that a chiral assembly was formed in the gel.
Ikeda, Toshiaki; Hirano, Kyohei; Haino, Takeharu, Mater. Chem. Front., 2018, 2, 468-474(Front Cover)