文献
2025
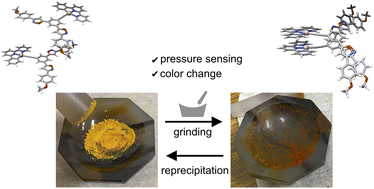
●Mechano-responsive color changes of a Pt(ii) complex possessing triethylene glycol towards pressure sensors
Mechanochromic molecules have attracted significant attention owing to their potential in the development of pressure sensors. However, relatively few studies have investigated the detailed mechanisms of the mechano-responsive nature and the quantitative visualization of mechanical forces. Herein, we report a square-planar platinum complex possessing triethylene glycol chains that exhibits mechanocromic behavior in the amorphous phase. Its mechanochromic nature was established using a combination of spectroscopic techniques, powder X-ray diffraction analyses, and computational chemistry techniques. The continuous changes in emission intensity allowed the platinum complex to be used as a mechanical force sensor, where the output signals were readable using a luminescence spectrometer. These findings demonstrate the potential benefits of square-planar platinum complexes and triethylene glycol chains for the creation of mechanochromic material.
Masaya Yoshida, Takehiro Hirao, Shin-ichi Kihara, Takeharu Haino, RSC Adv., 2025, 15, 21401–21407
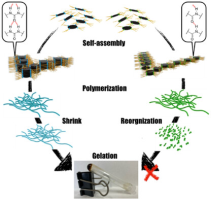
●Supramolecular Aggregates of Amide- and Urea-Functionalized Nanographene
Controlling the morphology of supramolecular nanographene(NG) aggregates is challenging. This study confirms that amideand urea-functionalized NG undergo self-assembly to form supramolecular aggregates with a morphology that depends on the incorporated functional group. Amide-functionalized NG forms stacked aggregates, whereas urea-functionalized NG organizes into network polymers. These distinct morphologies suggest that amide groups drive NG stacking, whereas urea groups support NG vertically and horizontally, likely owing to differences in the strengths of single and bifurcated N─H/O hydrogen bonds. Moreover, the functional group incorporated into NG influences the gelation properties of the system. Among the two tested systems, only urea-functionalized NG formed organogels, possibly because urea–urea hydrogen bonds, enable solvent-molecule trapping inside the network polymers formed in these NG systems. Thus, hydrogen bonds can regulate the morphology and function of supramolecular NG aggregates.
Haruka Moriguchi, Ryo Sekiya, Takeharu Haino, ChemistryEurope, 2025, 3, e2500015.
doi.org/10.1002/ceur.202500015
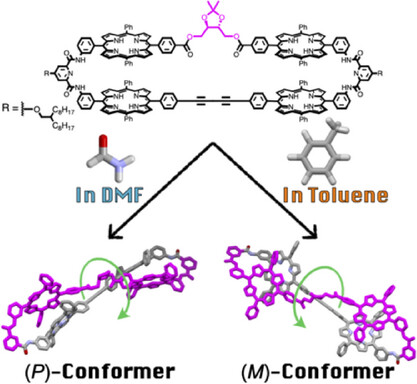
●Solvent-Directed Handedness in a Chirally Twisted Tetrakisporphyrin Macrocycle
A chirally twisted tetrakisporphyrin macrocycle was synthesized by incorporating a chiral dioxolane into a tetrakisporphyrin macrocycle. The solvent type influenced the preferred handedness of the twisted conformation. Circular dichroism measurements and computational analyses determined the handedness of the conformers in solvents toluene and dimethylformamide.
Kouta Tanabe, Naoyuki Hisano, Takeharu Haino, Asian J. Org. Chem. 2025, Accepted
doi.org/10.1002/ajoc.202500251
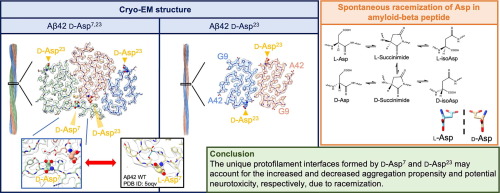
●Impacts of D-aspartate on the Aggregation Kinetics and Structural Polymorphism of Amyloid β Peptide 1–42
Isomerization of L-Aspartate (L-Asp) into D-aspartate (D-Asp) occurs naturally in proteins at a rate that is much faster than that of other amino acid types. Accumulation of D-Asp is age-dependent, which could alter protein structures and, therefore, functions. Site-specific introduction of D-Asp can accelerate aggregation kinetics of a variety of proteins associated with misfolding diseases. Here, we showed by thioflavin T fluorescence that the isomerization of L-Asp at different positions of amyloid β peptide 1–42 (Aβ42) generates opposing effects on its aggregation kinetics. We further determined the atomic structures of Aβ42 amyloid fibrils harboring a single D-Asp at position 23 and two D-Asp at positions 7 and 23 by cryo-electron microscopy helical reconstruction – cross-validated by cryo-electron tomography and atomic force microscopy – to reveal how D-Asp7 contributes to the formation of a unique triple stranded amyloid fibril structure stabilized by two threads of well-ordered water molecules. These findings provide crucial insights into how the conversion from L- to D-Asp influences the aggregation propensity and amyloid polymorphism of Aβ42.
Li-Ching Hsiao, Chih-Hsuan Lee, Karine Mazmanian, Masaya Yoshida, Genta Ito, Takuya Murata, Naoko Utsunomiya-Tate, Takeharu Haino, Shih-ichi Tate, Shang-Te Danny Hsu, Journal of Molecular Biology, 2025, 437, 169092
doi.org/10.1016/j.jmb.2025.169092
●Helical Supramolecular Polymers Formed via Head-to-Tail Host-Guest Complexation of Chiral Bisporphyrin Monomers with Trinitrofluorenone
The intermolecular host-guest complexation of head-to-tail monomers consisting of cleft-shaped bisporphyrin and trinitrofluorenone units connected by a chiral binaphthyl linker was employed to construct helically twisted supramolecular polymers. Results from 1H NMR, diffusion-ordered NMR spectroscopy, and viscometry experiments revealed that the supramolecular polymerization of these monomers follows a ring-chain competition mechanism. The introduction of bulky substituents at the linker significantly suppressed the formation of macrocyclic oligomers, whereas smaller alkyl chains facilitated the formation of the cyclic form. The chirally twisted structures of the supramolecular polymers were confirmed using circular dichroism spectroscopy. Atomic force microscopy revealed that the (R)- and (S)-configurations of the binaphthyl linkers induced right- and left-handed helical structures, respectively, in the supramolecular polymer chains. The absence of cooperativity in the supramolecular copolymerization of (R)- and (S)-1a resulted in the formation of stereo-random supramolecular copolymers.
Naoyuki Hisano, Tomoki Kodama, Soichiro Koya, Takeharu Haino, Chem. Eur. J., 2025, 31, e202404210.(Very Important Paper)
●Chirality Generation on Carbon Nanosheets by Chemical Modification
Chirality is an intriguing property of molecules, and an exciting area of study involves the generation of chirality in nanographene (NGs), also known as graphene quantum dots. Unlike those synthesized through stepwise carbon-carbon bond formation by organic reactions (bottom-up method), NGs are obtained by cutting parent carbons (top-down method) pose challenges in precisely regulating their three-dimensional structures by post-synthesis. This includes the incorporation of non-hexagonal rings and helicene-like structures in carbon frameworks. Currently, edge functionalization is the only method for generating chirality in NGs produced by the top-down method. While various chiral NGs have been synthesized through organic methods, examples of chemical modification remain rare due to limited structural information and the substantial size of NGs. However, these problems can be mitigated by disclosing the structures of NGs, particularly their edge structures. This minireview focuses on recently published papers that address the structural characterization of NGs and their chirality generation by edge modification. Comparing these NGs with those synthesized by organic synthesis will help to develop reasonable strategies for creating sophisticated chiral NGs. We hope this mini-review contributes to the advancement of NG-organic hybrid materials.
Ryo Sekiya, Saki Arimura, Haruka Moriguchi, Takeharu Haino, Nanoscale, 2025, 17, 774-787.
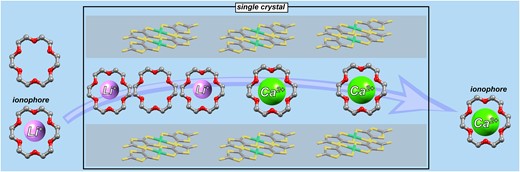
●Single-crystal-to-single-crystal transformation based on ionophore-like transport
We have already reported that Li2([18]crown-6)3[Ni(dmit)2]2(H2O)4 crystals, which have an ion channel structure, can be reversibly converted to Ca([18]crown-6)[Ni(dmit)2]2(H2O)3 crystals by ion exchange while maintaining their single-crystal state. In this process, inorganic ions as well as crown ethers traverse in and out of the crystal. In this study, [18]crown-6-d4, a partially deuterated [18]crown-6, was used to investigate the behavior of inorganic ions and crown ethers during ion exchange and found that Li+-[18]crown-6 supramolecules could act as ionophores in the crystal.
Mizuki Ito, Jun Manabe, Katsuya Inoue, Takehiro Hirao, Takeharu Haino, Tomoyuki Akutagawa, Kiyonori Takahashi, Takayoshi Nakamura, Sadafumi Nishihara, Chem. Lett., 54, 2025, upae252.
doi.org/10.1093/chemle/upae252
●Controlled Helical Organization in Supramolecular Polymers of Pseudo-Macrocyclic Tetrakisporphyrins
Tetrakisporphyrin monomers with amino acid side chains at each end form intramolecular antiparallel hydrogen-bonds to adopt chirally twisted pseudo-macrocyclic structures that result in right-handed and left-handed (P)- and (M)-conformations. The pseudo-macrocyclic tetrakisporphyrin monomers self-assembled to form supramolecular helical pseudo-polycatenane polymers via head-to-head complementary dimerization of the bisporphyrin cleft units in an isodesmic manner. The formation of one-handed supramolecular helical pseudo-polycatenane polymers was confirmed by circular dichroism spectroscopy. The methyl and iso-propyl groups at the stereogenic center greatly enhanced the induced circular dichroism (CD) in the Soret bands of the supramolecular helical pseudo-polycatenane polymers. The induced CDs were reduced upon the introduction of large iso-butyl and tert-butyl groups. Atomic force microscopy revealed well-grown and long supramolecular helical pseudo-polycatenane polymer chains with chain lengths in the range of 361 to 13.6 nm. The right-handed helical chains were established by the self-assembly of the right-handed (P)-conformation of the pseudo-macrocyclic monomer.
Naoka Fujii, Naoyuki Hisano, Takehiro Hirao, Shin-ichi Kihara, Kouta Tanabe, Masaya Yoshida, Shin-ichi Tate, Takeharu Haino, Angew. Chem. Int. Ed. 2025, 64, e202416770(Hot Paper, Inside Back Cover)
●Temperature-Dependent Left- and Right-Twisted Conformational Changes in 1:1 Host-Guest Systems: Theoretical Modeling and Chiroptical Simulations
An efficient strategy for high-performance chiral materials is to design and synthesize host molecules with left- and right- (M- and P-) twisted conformations and to control their twisted conformations. For this, a quantitative analysis is required to describe the chiroptical inversion, chiral transfer, and chiral recognition in the host-guest systems, which is generally performed using circular dichroism (CD) and/or proton nuclear magnetic resonance (1H-NMR) spectroscopies. However, the mass-balance model that considers the M- and P-twisted conformations has not yet been established. In this study, we derived the novel equations based on the mass-balance model for the 1:1 host-guest systems. Then, we further applied them to analyze the 1:1 host-guest systems for the achiral calixarene-based capsule molecule, achiral dimeric zinc porphyrin tweezer molecule, and chiral pillar[5]arene with the chiral and/or achiral guest molecules by using the data obtained from the CD titration, variable temperature CD (VT-CD), and 1H-NMR experiments. The thermodynamic parameters (ΔH and ΔS), equilibrium constants (K), and molar CD (Δε) in the 1:1 host-guest systems could be successfully determined by the theoretical analyses using the derived equations.
Nozomu Suzuki, Daisuke Taura, Yusuke Furuta, Yudai Ono, Senri Miyagi, Ryota Kameda, Takeharu Haino, Angew. Chem. Int. Ed. 2025, 64, e202413340.
2024
●Chiral Induction of a Tetrakis(porphyrin) in Various Chiral Solvent
Non-covalent interactions offer an alternative way for developing stimulus-responsive materials such as sensors, machines, and drug-delivery systems. We recently reported that a urethane-equipped tetrakis(porphyrin) forms one-handed helical supramolecular polymers in solution in response to chirality of chiral solvents. Conformational changes in helical sense were detected using circular dichroism (CD) spectroscopy, which showed that the tetrakis(porphyrin) can possibly be used as a sensor for determining the enantiomeric excess of a chiral analyte. Hence, we studied the scope and limitations of the chiral-induction behavior of tetrakis(porphyrin) to deepen the understanding of tetrakis(porphyrin)-based chiral sensing systems. Herein, we report the chiral-induction behavior of tetrakis(porphyrin) in various chiral solvents, which was found to be CD-active in many chiral solvents. Notably, the tetrakis(porphyrin) was CD active in a cryptochiral molecular solvent, which is exciting because the chiralities of acyclic saturated hydrocarbons are hard to sense. Consequently, this study highlights the potential advantages of supramolecular chiral sensors capable of targeting a wide range of analytes, including molecules that are absorption-silent in the UV/vis region, ones devoid of anchoring functional groups, and acyclic, saturated hydrocarbons.
Takehiro Hirao, Sei Kishino, Masaya Yoshida, Takeharu Haino, Chem. Eur. J., 2024, 30, e20240356.
●Assessment of Edge Modification of Nanographene
Carboxy groups on the edges of nanographene (NG) enable functionalization for realizing NG-organic hybrid materials. Therefore, assessment of the edge-functionalization of the electronic structures of NGs is valuable for the rational design of functional carbon materials. In this study, the structures of model NGs comprising 174 carbon atoms with armchair edges and various functional groups at the edges were computed. To achieve the greatest possible similarity between the computed structure and the real one, the carbon framework was designed based on experimental observations. The functional groups can be accessed via suitable chemical reactions. The computations predicted that although the conversion of carboxyl groups with electron-withdrawing/donating groups influences the orbital energies, the HOMO-LUMO (H-L) gap is not significantly affected, except in a few cases. Among the evaluated examples, π-extension had the greatest influence on the H-L gap. Interestingly, for the Pd2+-coordinated NG, the participation of the low-lying LUMO localized on Pd2+ in the surface-to-metal transitions seemingly narrowed the H-L gap, and a surface-to-ligand transition was observed.
Ryo Sekiya and Takeharu Haino, ChemPysChem, 2024, 30, e202402922.(Front Cover)
●Latent Porosity of Planar Tris(phenylisoxazolyl)benzene
Interest in developing separation systems for chemical entities based on crystalline molecules has provided momentum for the fabrication of synthetic porous materials showing selectivity in molecular encapsulation, such as metal-organic frameworks, covalent organic frameworks, hydrogen-bonded organic frameworks, zeolites, and macrocyclic molecular crystals. Among these, macrocyclic molecular crystals have generated renewed interest for use in separation systems. Selective encapsulation relies on the sizes, shapes, and dimensions of the pores present in the macrocyclic cavities; thus, nonmacrocyclic molecular crystals with high selectivity for molecular encapsulation via porosity-without-pore behaviors have not been studied. Here, we report that planar tris(phenylisoxazolyl)benzene forms porous molecular crystals possessing latent pores exhibiting porosity-without-pore behavior. After exposing the crystals to complementary guest molecules, the latent pores encapsulate cis- and trans-decalin while maintaining the structural rigidity responsible for the high selectivity. The encapsulation via porosity without pores is a kinetic process with remarkable selectivity for cis-decalin over trans-decalin with a cis-/trans-ratio of 96:4, which is confirmed by single-crystal X-ray diffraction and powder X-ray diffraction analyses. Hirshfeld surface analysis and fingerprint plots show that the latent intermolecular pores are rigidified by intermolecular dipole‒dipole and π–π stacking interactions, which determines the remarkable selectivity of molecular recognition.
Yudai Ono, Takehiro Hirao, Naomi Kawata, and Takeharu Haino, Nat. Commun., 2024, 15, 8314.
DOI:10.1038/s41467-024-52526-9
●Conformation Regulation of Trisresorcinarene Directed by Cavity Solvation
This compound is a synthetic macrocycle comprising three pivaloyl-protected resorcinarene units connected by six pentylene chains. We conducted a conformational study using 1H-NMR, X-ray diffraction (XRD), and computational analyses. The macrocycle adopts two conformers, one open, the other closed. The ratio of the open to closed forms depended on the solvent used. Only the open form existed in [D8]toluene, both forms coexisted in [D6]benzene, and the closed form was the major conformer in [D1]chloroform. The benzene-solvated open form observed in the solid state suggests that cavity solvation by solvent molecules directs the open form. The open form was the major or only conformer in [D10]o- and [D10]m-xylene and [D12]mesitylene, whereas the closed form was the major conformer in [D6]acetone. The open and closed forms were equally populated in [D10]p-xylene, suggesting that the size, shape, and dimensions of the solvent molecules most likely influenced the conformation of the protected trisresocinarene.
Daisuke Shimoyama, Ryo Sekiya, Shoichiro Inoue, Naoyuki Hisano, Shin-ichi Tate, and Takeharu Haino, Chem. Eur. J. 2024, 30, e202402922.(Front Cover)
●Spectrally Selective Leakage of Light from Self-Assembled Supramolecular Nanofiber Waveguides Induced by Surface Plasmon Polaritons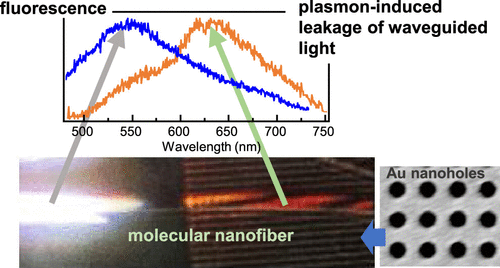
We report surface plasmon polariton (SPP)-induced spectrally selective leakage of light waveguided in supramolecular nanofibers. The nanofibers are fabricated by self-assembly of tris(phenylisoxazolyl)benzene derivative molecules, and their diameter ranges from nanometers to hundreds of nanometers. Nanofibers with heights of more than 200 nm are shown to function as waveguides for fluorescence excited in one location by a focused 360 nm laser. The fluorescence can transfer the whole length of the nanofibers of tens of micrometers and is outcoupled from the nanofiber ends. The waveguiding phenomenon dramatically changes when the nanofibers are deposited on SPP-generating substrates. The substrates in the form of nanohole arrays are fabricated on a gold film with a pitch of 500 nm, a diameter of 250 nm, and a depth of 40 nm. On the SPP substrates, the nanofiber waveguides exhibit strong leakage of the guided light. The spectrum of the leaked light is consistent with the SPP resonance wavelength, and its polarization corresponds to the TE waveguided mode. We propose mechanisms of the observed phenomena that include either excitation of the SPPs via the waveguide evanescent field or direct enhancement of the leakage by the modified density of states near the plasmonic substrate.
Qiwen Tan, Nao Koishihara, Shun Omagari, Takehiro Hirao, Takeharu Haino, Martin Vacha, J. Phys. Chem. C 2024, 128, 10, 4295–4302.
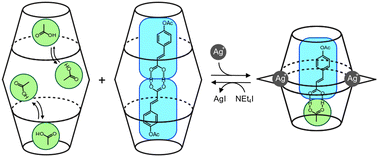
●Selective encapsulation of carboxylic acid dimers within a size-regulable resorcinarene-based hemicarcerand
A cavity within a resorcinarene-based hemicarcerand was contracted and expanded through conformational changes induced by the complexation and decomplexation, allowing self-sorting of homo- and heterodimeric carboxylic acid pairs.
Kentaro Harada, Yudai Ono, Ryo Sekiya, and Takeharu Haino, Chem. Commun. 2024, 60, 6603(Front Cover)
●Synthesis of Supramolecular Polymers
Takehiro Hirao, Masaya Yoshida, and Takeharu Haino, Handbook of Functional Polymers, Chapter 40: Supramoelcule, Y. Chujo Ed., Springer.
●Supramolecular Synthesis of Star Polymers
Supramolecular polymers, in which monomers are assembled via intermolecular interactions, have been extensively studied. The fusion of supramolecular polymers with conventional polymers has attracted the attention of many researchers. In this review article, the recent progress in the construction of supramolecular star polymers, including regular star polymers and miktoarm star polymers, is discussed. The initial sections briefly provide an overview of the conventional classification and synthesis methods for star polymers. Coordination-driven self-assembly was investigated for the supramolecular synthesis of star polymers. Star polymers with multiple polymer chains radiating from metal–organic polyhedra (MOPs) have also been described. Particular focus has been placed on the synthesis of star polymers featuring supramolecular cores formed through hydrogen-bonding-directed self-assembly. After describing the synthesis of star polymers based on host–guest complexes, the construction of miktoarm star polymers based on the molecular recognition of coordination capsules is detailed.
Takeharu Haino and Natsumi Nitta, ChemPlusChem, 2024, 89, e202400014.(Front Cover)
●Kinetic Resolution of Secondary Alcohols Catalyzed at the Exterior of Chiral Coordinated Capsules
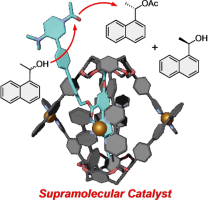
Confined spaces inside molecular hosts can function as reaction vessels. However, this concept significantly limits the scope of reactants. When the exterior of molecular hosts is used instead, we can ease the restriction because reactants are not necessary to be trapped inside molecular hosts, although studies along this line have not been reported. As a proof-of-concept of enantioselective reactions at the exterior of chiral molecular hosts, we utilized host‒guest complexes of enantiomerically enriched Cu-coordinated capsules and guests possessing a catalytic center to realize the kinetic resolution of secondary alcohols. Under suitable reaction conditions, a selectivity factor of 2.6 was realized, demonstrating that the reactions occur at the exterior of the capsules. A series of experiments indicated that the substituents on the 2,2’-bipyridyl arms and the alkyl chains on the lower rim contributed to the enantioselectivity of the reactions.
Kentaro Harada, Ryo Sekiya, Takeharu Haino, Chem. Eur. J., 2024, 30, e202304244.(Front Cover)
●Application of Exciton Coupling for Characterization of Nanographene Edge
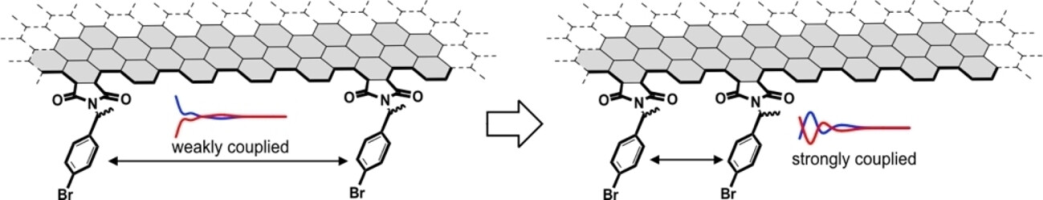
The structural characterization of nonstoichiometric nanographenes (NG)-organic hybrid materials is usually difficult. The number of substituents on the edge and their arrangements are frequently questioned but are difficult to answer. Since the number of functional groups is closely related to the distance between the nearest neighbors (dISD), the extraction of dISD from spectroscopic data could provide important information on their structural characterization. We show that exciton coupling, which is a theoretical prediction of the absolute structures of discrete molecules, is a possible candidate to address this issue. The comparison of the calculated CD spectra of the chiral chromophores extracted from the model NG edge with the observed edge spectra indicated a dISD of ca. 8 Å; this corresponded to substitution on every other armchair edge. Furthermore, an up-up-down-down alternate orientation was found to be a possible edge structure. Although the procedure was limited to NGs carrying chiral substituents, our method could facilitate the detailed structural characterization of NG-organic hybrid materials.
Ryo Sekiya and Takeharu Haino, ChemPhysChem, 2024, 25, e202300740.(Front Cover)
●Synthesis and Cooperative Guest Binding of Tetrameric Porphyrin Macrocycle
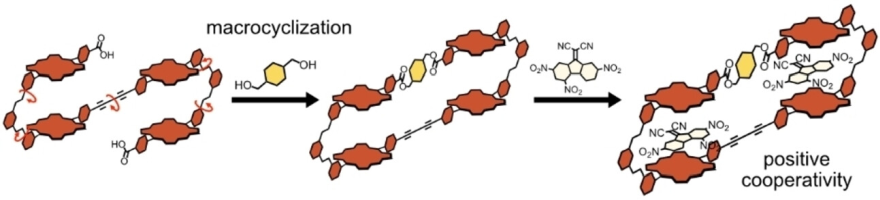
A new family of macrocyclic host molecules that possess two bisporphyrin
clefts was synthesized. The tetrameric porphyrin macrocycle provides the
two guest binding sites in which two electron-deficient aromatic molecules
are encapsulated through donor–acceptor and π-π stacking interactions.
The tetrameric porphyrin macrocycle showed positive cooperativity, whereas
the nonmacrocyclic analog did not. The preorganization concept drives positive
cooperativity, which is directed by the entropy benefit to macrocyclization.
Kouta Tanabe, Naoyuki Hisano, and Takeharu Haino, ChemistrySelect, 2024, 9, e202305211.
●Intermediate Color Emission via Nanographenes with Organic Fluorophores

Photoluminescence (PL) color can be tuned by mixing fluorophores emitting the three primary colors in an appropriate ratio. When color tuning is achieved on a single substrate, we can simplify device structures. We demonstrated that nanographenes (NGs), which are graphene fragments with a size of tens of nanometers, could be utilized as carriers of fluorophores. The addition of red- and blue-light-emitting fluorophores on the edge successfully reproduced the purple light. The relative PL intensities of the fluorophores could be regulated by the excitation wavelength, enabling multicolor emission between blue and red light. Owing to the triphenylamine units of the fluorophores, the NGs showed PL enhancement due to aggregation. This characteristic was valuable for the fabrication of solid polymer materials. Specifically, the functionalized NGs can be dispersed into polyvinylidene difluoride. The resultant polymer films emitted red, blue, and purple color. Our study demonstrated the potential applicability of NGs for fluorophore carriers capable of reproducing intermediate colors of light.
Saki Arimura, Ikuya Matsumoto, Ryo Sekiya, and Takeharu Haino, Angew. Chem. Int. Ed., 2024, 63, e202315508.(Frontispiece)
●Supramolecular Polymerization Behavior of a Ditopic Self-folding Biscavitand
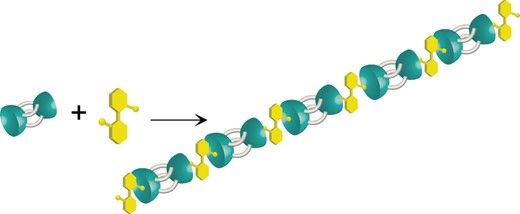
Reported herein is the supramolecular polymerization of a mixture of a feet-to-feet connected biscavitand and a homoditopic quinuclidinium guest that is regulated by cooperativity in the host–guest association. The diffusion ordered NMR spectroscopy (DOSY) was used to evaluate the supramolecular polymerization in toluene, CHCl3, and THF. Upon concentrating the solutions of the biscavitand with the quinuclidinium guest in CHCl3 and THF, the diffusion coefficient (D) values were meaningfully decreased, indicating that the host-guest complexation facilitated the supramolecular polymerization. In contrast, the slight change of the D value in toluene suggests that the supramolecular polymerization was suppressed, although the binding constant (K) between the cavitand and quinuclidinium guest was reported to be 105 L mol–1 in toluene. The viscosity measurements showed both the critical polymerization concentration (CPC) and entangled concentration (Ce) upon concentrating the CHCl3 solution of the mixture. Neither the CPC nor Ce was seen in the toluene solution of the mixture. Accordingly, the strong negative cooperativity in the 1:2 host-guest complexation of the biscavitand discouraged the supramolecular polymerization in toluene. These findings are valuable to deepen the understanding of the host–guest association-driven supramolecular polymerization behaviors regulated by a combination of cooperativity and K value in solution.
Haruna Fujimoto, Takehiro Hirao, and Takeharu Haino, Bull. Chem. Soc. Jpn, 2024, 97, uoad016.
2023
●Molecular Recognition Process in Resorcinarene-based Coordination Capsules

Cu and Ag capsules can take up various organic molecules. Their molecular recognition possibly involves partial dissociation and slippage. We investigated molecular recognition processes in Cu and Ag capsules by CD and 1H NMR spectroscopy and employed 4,4’-diacetoxy biphenyl carrying two benzothiadiazole groups as a probe. CD and 1H NMR measurements reveal that the host-guest complexation proceeds under second-order reactions and that these capsules undergo partial dissociation to take up the probe in [D1]chloroform and [D8]THF. Slippage also contributes to host-guest complexation for a Cu capsule that carries p-methoxyphenyl groups on the 2,2’-bipyridiyl arms. DFT calculations suggest that π/π stacking interactions between the electron-rich p-methoxyphenyl group and the electron-poor 2,2’-bipyridyl arm elongate the capsule, allowing the guest to access the cavity without partial dissociation.
Kentaro Harada, Ryo Sekiya, and Takeharu Haino, Chem. Eur. J., 2023, 29, e202302581.
●A robust heterodimeric bis-Rh(iii)–porphyrin macrocycle for the self-assembly of a kinetically stable [2]-rotaxane

We report the self-assembly of a robust di-nuclear tetralactam macrocyle based on two symmetric components: a Rh(III) bis-porphyrin and a bis-pyridyl ligand. We probe the binding properties of the tetralactam macrocycle with adipamide derivatives using 1H NMR spectroscopy. On the one hand, we show that the binding of the adipamide having linear alkyl chains that can thread through the intact macrocycle's cavity produces a weakly bound 1 : 1 complex stabilized by four intermolecular hydrogen bonds and featuring a preferred binding geometry of [2]pseudorotaxane topology. On the other hand, we detect the formation of two different complexes in the binding of an analogous adipamide possessing bulky stoppers (dumb-bell axle). The initial addition of the dumb-bell guest induces the formation of a 1 : 1 complex featuring fast exchange kinetics on the 1H chemical shift timescale and exo-cyclic (non-threaded) binding geometry. Notably, in the presence of a large excess of the dumb-bell guest and at suitable concentrations of the macrocycle (>5 mM) we observe the emergence of a second species displaying slow exchange kinetics. This observation allows the undisputed assignment of a [2]rotaxane topology to the second complex. The significant increase in kinetic stability featured by the di-nuclear Rh(III) [2]rotaxane complex contrasts with its reduction in thermodynamic stability (more than one order of magnitude) compared to the previously described di-nuclear Zn(II) counterpart.
Naoyuki Hisano, Virginia Valderrey, Gemma Aragay, Pablo Ballester, Dalton Trans., 2023, 52, 8344-8352.
●Induction of Chirality on Nanographenes

Chirality induction is an important topic in studies of nanographenes (NGs). We report chirality enhancements of NGs through postsynthetic chemical modifications of NGs with pyrene and m-terphenyl groups. These substituents were installed into N-(p-bromophenylethyl)imides on the edges of the NGs with Pd-catalyzed cross-coupling reactions. Circular dichroism (CD) spectra demonstrated that these bulky substituents improved the induced CD signal of the NGs compared to those previously reported and suggested that they induced the opposite chirality. Density functional theory calculations indicated possible edge structures for the NGs and indicated that π/π and CH/π interactions among the neighboring substituents influenced the orientations of the imides. These imides distorted the edges, and the distorted edges eventually generated the chiral environments of the NGs. The interactions among the substituents are, therefore, likely to allow detection of the CD signals in the visible region and induction of the opposite chirality.
Saki Arimura, Ikuya Matsumoto, Shohei Nishitani, Ryo Sekiya, and Takeharu Haino, Chem. Asian J., 2023, 18, e202300126.
●Cooperativity in molecular recognition of feet-to-feet-connected biscavitands
Octaphosphonate biscavitand and self-folding deep biscavitand show strong positive and negative cooperativity, respectively. The mechanism of the cooperativity is discussed in terms of thermodynamic parameters and the detailed structure of the host-guest complexes. The two cavitand units of both biscavitands are tightly connected via four butylene linkers; thus, they are conformationally coupled, with the first guest binding information transferred to the resting-state cavities. This preorganization modulates the successive guest binding process in strong positive and negative cooperative manners, even though they display structural similarity. The first guest complexation always preorganizes the resting-state cavities where an existing water cluster and a toluene molecule are enthalpically stabilized. Successive guest complexation competes with the water cluster or a toluene molecule, reducing enthalpy gains. However, the desolvation upon successive guest binding processes liberate the solvents within the resting-state cavities. The water cluster is composed of 12 water molecules that are released upon successive guest complexation, resulting in a large entropy benefit. In contrast, toluene desolvation results in a limited entropy benefit. The difference in entropy benefits directs the strong positive or negative cooperativity of the structurally similar biscavitands.
Takeharu Haino, Pure Appl. Chem., 2023, 95, 343-352.
●Negative Homotropic Cooperativity in Guest Binding of a Trisporphyrin Double Cleft
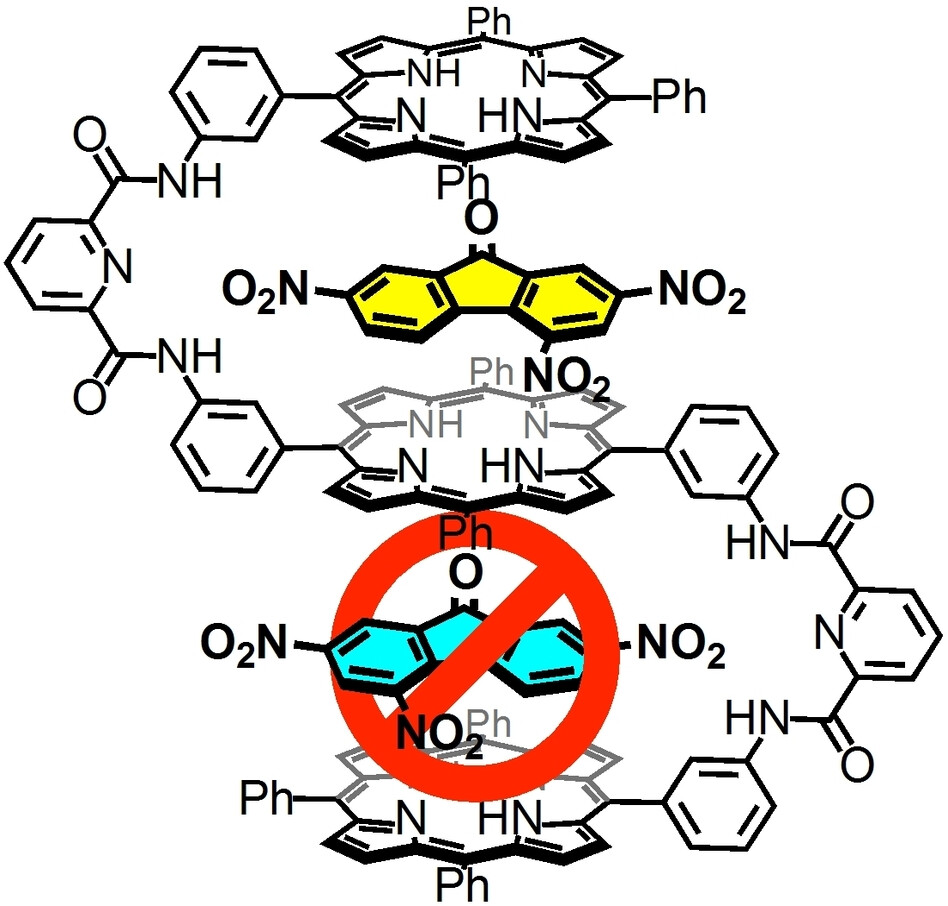 The negative homotropic allostery of a triple-layered trisporphyrin cleft
with two guest binding sites was confirmed. The X-ray crystal structures
of the 1:2 host-guest complexes showed that the trisporphyrin accommodated
two guest molecules within the cleft via π–π stacking and donor-acceptor
interactions. In solution, 1H NMR and Job plots showed 1:2 host-guest complexes.
Isothermal titration calorimetry (ITC) and UV/vis absorption spectroscopy
were employed to evaluate the binding constants and cooperativities. The
guest binding of the trisporphyrin showed negative cooperativity and noncooperativity
depending on the structures of the guest molecules. The correlations between
the interaction parameters (α) and Hill constants were determined. ITC
experiments showed that the host-guest complexation of trisporphyrin with
electron-deficient guests incurred an enthalpy penalty in the successive
guest binding process. DFT calculations revealed that the first guest binding
reduced the electron density of the central porphyrin plane, which led
to an energetic penalty that weakened the successive binding process.
The negative homotropic allostery of a triple-layered trisporphyrin cleft
with two guest binding sites was confirmed. The X-ray crystal structures
of the 1:2 host-guest complexes showed that the trisporphyrin accommodated
two guest molecules within the cleft via π–π stacking and donor-acceptor
interactions. In solution, 1H NMR and Job plots showed 1:2 host-guest complexes.
Isothermal titration calorimetry (ITC) and UV/vis absorption spectroscopy
were employed to evaluate the binding constants and cooperativities. The
guest binding of the trisporphyrin showed negative cooperativity and noncooperativity
depending on the structures of the guest molecules. The correlations between
the interaction parameters (α) and Hill constants were determined. ITC
experiments showed that the host-guest complexation of trisporphyrin with
electron-deficient guests incurred an enthalpy penalty in the successive
guest binding process. DFT calculations revealed that the first guest binding
reduced the electron density of the central porphyrin plane, which led
to an energetic penalty that weakened the successive binding process.
Naoyuki Hisano, Tomoki Kodama, and Takeharu Haino, Chem. Eur. J., 2023, 29, e202300107
●Effects of Edge Functionalization of Nanographenes with Small Aromatic Systems
Regulation of the physical properties of nanographenes (NGs) by edge functionalization is an active research area. We conducted a computational study of the effects of edge functionalization on the physical properties of NGs. The computed NGs were models of experimentally obtained NGs and composed of a C174 carbon framework with from one to four 3,5-dimethylnaphthalene units on the edge. The effects were assessed structurally, magnetically, and electronically by the least square planarity index, harmonic oscillator model of aromaticity, nucleus-independent chemical shift, and HOMO-LUMO (H-L) gaps. Density functional theory calculations indicated that although the structures of the model NGs were not very sensitive to edge functionalization, the magnetic and electronic properties were. The installed substituents narrowed the H-L gap and induced a redshift of the PL band by the π conjugation between NG and the substituent. These results were consistent with the extension of the absorption band and the redshift of the PL bands of the experimentally modified NGs. Furthermore, the calculations confirmed the contribution of the charge transfer character to the absorption spectra.
Shusaku Takahashi, Ryo Sekiya, and Takeharu Haino, ChemPhysChem, 2023, 24, e202300066.
●Nanoarchitectonics of Supramolecular Porphyrins Based on a Bis(porphyrin) Cleft Molecule
This account describes the construction of supramolecular constructs based on our bis(porphyrin) cleft molecule. The bis(porphyrin) cleft molecule was originally synthesized as a tweezer-shaped host molecule for planar guest molecules. A detailed study on the bis(porphyrin) cleft molecule revealed that the bis(porphyrin) cleft molecule forms two kinds of supramolecular structures. One structure is a self-complementary dimer obtained through intermolecular hydrogen bonding, and the other structure is a host-guest complex, in which the electron-rich cleft cavity accommodates electron-deficient guests through donor-acceptor interactions. Through the two supramolecular structures, two distinct supramolecular polymers can be formed through self-complementary dimerization or donor-acceptor host-guest complexation. The supramolecular chain structures were modified by judiciously using two distinct supramolecular structures. In the main text, several results, including the binding capability of our bis(porphyrin) cleft molecule, the formation of supramolecular porphyrin complexes, and the supramolecular polymerization behaviors of the bis(porphyrin) cleft molecule, are reported. In conclusion, the future direction of the bis(porphyrin) cleft molecule is provided.
Takehiro Hirao and Takeharu Haino, J. Porphyr. Phthalocyanines, 2023, 27, 966-979
●Substituent-Induced Supramolecular Aggregates of Edge Functionalized Nanographenes

Precisely controlled molecular assemblies often display intriguing morphologies and/or functions arising from their structures. The application of the concept of the self-assembly for controlling the aggregation of nanographenes (NGs) is challenging. The title NGs are those carrying both long alkyl chains and tris(phenylisoxazolyl)benzene (TPIB) on the edge. The former group secures the affinity of NGs for organic solvents, and the latter group drives the one-dimensional arrangement of NGs through the interactions between the TPIB units. The concentration-dependent and temperature variable 1H NMR, UV-vis, and PL spectra demonstrate the aggregation of NGs in 1,2-dichloroethane, and the aggregation is controllable by the regulation of the solvent polarity. AFM images give the stacked structures of the NGs, and these aggregates turn out to be network polymeric structures at a high concentration. These observations demonstrate that the synergy of the face-to-face interactions between the surfaces and the interactions between the TPIB units is effective for controlling the self-assembly of the NGs.
Haruka Moriguchi, Ryo Sekiya, and Takeharu Haino, Small, 2023, 19, 2207475.
●Synthesis of Supramolecular A8Bn Miktoarm Star Copolymers by Host–Guest Complexation

We report a brand-new synthetic method to construct supramolecular A8Bn (n = 1, 2, 4) miktoarm star copolymers via host–guest complexation between a resorcinarene-based coordination capsule possessing eight polystyrene chains and 4,4-diacetoxy biphenyl guest molecules keeping one, two and four polymethyl acrylate chains. The formation of the supramolecular A8Bn (n = 1, 2, 4) miktoarm star copolymers was confirmed by dynamic light scattering (DLS), size exclusion chromatography (SEC), and diffusion-ordered NMR spectroscopy (DOSY). Differential scanning calorimetry (DSC) measurements revealed that the miktoarm copolymers were phase-separated in bulk. The micro Brownian motion of the capsule surrounded by polymethyl acrylate arms and polystyrene arms in the A8B4 structure was markedly enhanced in bulk due to a weak segregation interaction between the immiscible arms.
Natsumi Nitta, Shin-ichi Kihara, Takeharu Haino, Angew. Chem. Int. Ed, 2023, 62, e202219001.
●Supramolecular Chiral Sensing by Supramolecular Helical Polymers
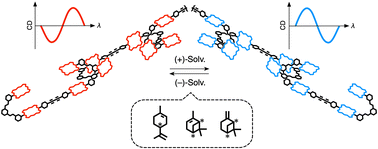
A tetrakis(porphyrin) with branched side chains self-assembled to form supramolecular helical polymers both in solution and in the solid state. The helicity of the supramolecular polymers was determined by the chirality of solvent molecules, which permitted the polymer chains to be used in chiral sensing.
Takehiro Hirao, Sei Kishino and Takeharu Haino, Chem. Comm, 2023, 59, 2421-2424.
●Lanthanide and Actinide Ion Complexes Containing Organic Ligands Investigated by Surface-Enhanced Infrared Absorption Spectroscopy
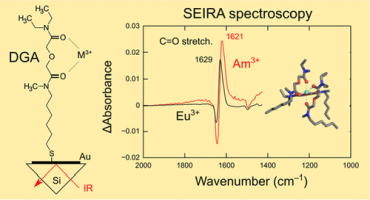
A new technique, surface-enhanced infrared absorption (SEIRA) spectroscopy, was used for the structural investigation of lanthanide (Ln) and actinide (An) complexes containing organic ligands. We synthesized thiol derivatives of organic ligands with coordination sites similar to those of 2-[N-methyl-N-hexanethiol-amino]-2-oxoethoxy-[N′,N′-diethyl]-acetamide [diglycolamide (DGA)], Cyanex-272, and N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN), which have been used for separating Ln and An through solvent extraction. These ligands were attached on a gold surface deposited on an Si prism through S–Au covalent bonds; the gold surface enhanced the IR absorption intensity of the ligands. Aqueous solutions of Ln (Eu3+, Gd3+, and Tb3+) and An (Am3+) ions were loaded onto the gold surface to form ion complexes. The IR spectra of the ion complexes were obtained using Fourier transform infrared spectroscopy in the attenuated total reflection mode. In this study, we developed a new sample preparation method for SEIRA spectroscopy that enabled us to obtain the IR spectra of the complexes with a small amount of ion solution (5 μL). This is a significant advantage for the IR measurement of radiotoxic Am3+ complexes. In the IR spectra of DGA, the band attributed to C═O stretching vibrations at ∼1630 cm–1 shifted to a lower wavenumber by ∼20 cm–1 upon complexation with Ln and An ions. Moreover, the amount of the red shift was inversely proportional to the extraction equilibrium constant reported in previous studies on solvent extraction. The coordination ability of DGA toward Ln and An ions could be assessed using the band position of the C═O band. The Cyanex-272- and TPEN-like ligands synthesized in this report also showed noticeable SEIRA signals for Ln and An complexes. This study indicates that SEIRA spectroscopy can be used for the structural investigation of ion complexes and provides a microscopic understanding of selective extraction of Ln and An.
Sakiko Hirata, Ryoji Kusaka, Shogo Meiji, Seita Tamekuni, Kosuke Okudera, Shoken Hamada, Chihiro Sakamoto, Takumi Honda, Kosuke Matsushita, Satoru Muramatsu, Takayuki Ebata, Daisuke Kajiya, Ken-ichi Saitow, Toshiaki Ikeda, Takehiro Hirao, Takeharu Haino, Masayuki Watanabe, and Yoshiya Inokuchi, Inorg.Chem., 2023, 62, 1, 474–486.
DOI:10.1021/acs.inorgchem.2c03618
●Computational Studies on the Structures of Nanographenes with Various Edge Functionalities

Computational studies have often been carried out on hydrogen-terminated nanographenes (NGs). These structures are, however, far from those deduced from experimental observations, which have suggested armchair edges with two carboxy groups on the edges as dominant. We conducted computational studies on NGs consisting of C42, C60, C78, C96, C142, and C174 carbon atoms with hydrogen, carboxy, and N-methyl imide-terminated armchair edges. DFT calculations inform distorted basal planes and similar HOMO-LUMO gaps, indicating that the edge oxidation and functionalization do not very influence the electronic structure. Comparison of observed UV-vis spectra of carboxy- and N-octadecyl chain terminated NGs with calculated spectra of model NGs informs the contribution of pi-pi* transitions on the basal plane to the absorptions in the visible region. A dimeric structure of NG and octadecyl-installed NG demonstrate that both the distorted basal planes and the steric contacts among the functional groups widen the surface-to-surface distance thereby allowing the invasion of solvent molecules between the surfaces. This picture is consistent with the improved solubility of edge-modified NGs.
Shusaku Takahashi, Ryo Sekiya, and Takeharu Haino, ChemPhysChem, 2023, e202200465.
2022
●Development of Supramolecular Polymers with Unique Chain Structures
Takehiro Hirao, Takeharu Haino, Supramolecular Nanotechnology: Advanced Design of Self-Assembled Functional Materials, edited by O. Azzaroni and M. Conda-Sheridan, in Press, VCH-Wiley, Weinheim (ISBN-10: 3527349480)
●Macromolecular architectures constructed by biscalix[5]arene-[60]fullerene host-guest interactions
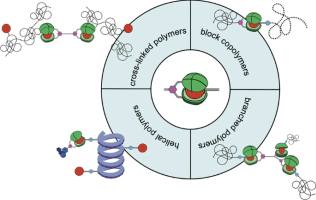
This focus review is designed to summarize the development of macromolecular architectures formed via biscalix[5]arene–[60]fullerene host–guest interactions. Biscalix[5]arene–fullerene host–guest complexation leads to various macromolecular architectures, including block polymers, star-shaped polymers, cross-linked polymers, and one-handed helical polymers, and host–guest complexation is not prevented by the long polymer chains owing to the high binding affinity between biscalix[5]arene and fullerene. These macromolecular architectures exhibited state-switching natures in response to environmental stimuli. Notably, one of them displayed behavior concordant with those of corresponding covalently linked polymers, including solution viscosity and thermal properties, even though the structures were maintained by relatively weak noncovalent interactions. These demonstrations indicate that biscalix[5]arene–[60]fullerene host–guest interactions can be used to create supramolecularly connected macromolecular architectures that can convert between assembled and disassembled states because of the dynamic nature of noncovalent interactions.
Takehiro Hirao, Polymer Journal, 2022, accepted.
DOI: 10.1038/s41428-022-00732-x
●Improved synthesis of tetrakis(porphyrin) molecular cleft via palladium-mediated cross-coupling between bis(porphyrin) boronic ester and bis(iodophenyl)butadiyne

A tetrakis(porphyrin) was prepared by Suzuki-Miyaura cross coupling between a bis(porphyrin) boronate ester and 1,4-bis(4-iodophenyl)-1,3-butadiyne. The overall yield of the tetrakis(porphyrin) was improved to be more than double the yield preciously reported. The successful preparation of the tetrakis(porphyrin) was characterized by a combination of NMR and HRMS techniques. Furthermore, HRMS evidenced the formation of supramolecular polymeric species driven by bis(porphyrin)-bis(porphyrin) dimerization in the gas phase.
Naoyuki Hisano, Takehiro Hirao, Kouta Tanabe, and Takeharu Haino, J. Porphyr. Phthalocyanines, 2022, 26, 683-689.
DOI: 10.1142/S1088424622500523
●Metal Nanoparticles on Lipophilic Nanographenes

Utilization of graphitic domains on nanographenes (NGs) for anchoring metal nanoparticles (NPs) can open the door for their applications in catalysis. Reported examples employ hydrophilic graphene oxides as substrates, making it difficult to coexist with organic substrates in organic solvents. The title NGs are metal NP-doped lipophilic NGs (M-NG1). Various metal cations form NPs with a diameter of a few to tens of nanometers on the basal plane. Owing to the lipophilic nature of NG1, M-NG1 is prepared by the reduction of the basal plane with sodium in THF followed by the addition of metal salts. Au-NPs on NG1 allow anchoring an organic thiol carrying an anthracene fluorophore, which is a proof of concept of composite materials utilizing the surface of NGs. The assessment of the catalytic function of Pd-NG1 reveals that chlorobenzene and bromobenzene yield a coupling product, and fluorobenzene also undergoes the reactions, demonstrating the catalytic function of Pd-NG1.
Shusaku Takahashi, Ryo Sekiya, and Takeharu Haino, Angew. Chem. Int. Ed., 2022, 61, e202205514.(Hot Paper)
●Chirality Induction on a Coordination Capsule for Circularly Polarized Luminescence

By utilizing a confined space inside a coordination capsule consisting of achiral components, we achieve trimeric structures composed of acetic acid and 2,3-disubstituted tartaric acid derivatives. Steric and electronic interactions between the substituents on the tartaric acid and 2,2′-bipyridyl arms of the capsule permit the transfer of the chirality of the tartaric acid to the capsule, resulting in diastereoenrichment of the host-guest complexes of up to 92 % de. The chiral templates can be washed away with diethyl ether, leaving an enantiomerically enriched capsule. The resulting capsule biases the dynamic axial chirality of a 4,4′-diacetoxybiphenyl guest carrying benzothiadiazole units, demonstrating guest-to-capsule and capsule-to-guest chirality transfer. The induced chirality on the bound guest enables it to emit circularly polarized luminescence in the NIR region, demonstrating the application of induced chirality for confined spaces for the generation of chiroptical properties.
Kentaro Harada, Ryo Sekiya, and Takeharu Haino, Angew. Chem. Int. Ed., 2022, 61, e202209340.
●共沸化合物を簡単に分離する。ー繰り返し使える吸着材料ー
(解説)平尾岳大(Hirao Takehiro), 月刊「化学」2022年8月号, p64-65.
●Self-assembly of neutral platinum complexes controlled by thermal inputs
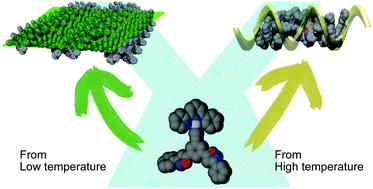
In this report, we describe the self-assembly behavior of neutral platinum complexes in toluene. The platinum complexes were seen to form two different types of assemblies depending on the preparation temperature.
Masaya Yoshida, Takehiro Hirao, and Takeharu Haino, Chem. Commun., 2022, 58, 8356-8359.
●Integration of Nanographenes and Organic Chemistry – Toward Nanographene-based Two-Dimensional Materials
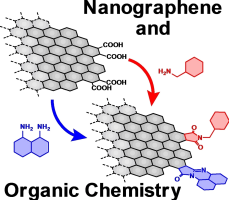
Graphene and its relatives have received considerable attention from the
fields of physics and chemistry since the isolation of pristine graphene
sheets. Nanographenes (NGs) are graphene fragments that are a few to tens
of nanometers in diameter. Compared to graphene and its relatives, such
as graphene oxides, NGs can be handled more easily, and their large surface
and oxygen functional groups on the edge allow postsynthetic modifications.
The study of NGs is gradually shifting from the development of synthetic
procedures to postsynthetic modification. From the structural point of
view, NGs can be regarded as two-dimensional carbon polymers. Their unique
structures and affinity for organic molecules make NGs excellent scaffolds
for two-dimensional materials, which are now an important topic in organic
and polymer chemistry. In this conceptual article, we introduce the position
of NGs from the perspective of two-dimensional substances and briefly review
both the structural features of NGs and the effects of functionalization
on their physical properties. These are valuable when producing reasonable
strategies for their postsynthetic modifications.
Ryo Sekiya and Takeharu Haino, ChemPhysChem, 2022, 23, e202200311.(Very Important Paper, Front Cover)
●Supramolecular Ensembles Formed via Calix[5]arene-Fullerene Host-Guest Interactions
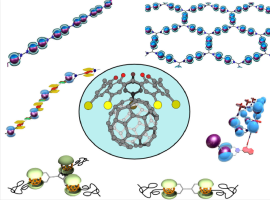
This minireview introduces the research directions for the synthesis of supramolecular fullerene polymers. First, the discovery of host-guest complexes of pristine fullerenes is briefed. We focus on progress in supramolecular fullerene polymers directed by the use of calix[5]arene-fullerene interactions, which comprise linear, networked, helical arrays of fullerenes in supramolecular ensembles. The unique self-sorting behavior of right-handed and left-handed helical supramolecular fullerene arrays is discussed. Thereafter, an extensive investigation of the calix[5]arene-fullerene interaction for control over the chain structures of covalent polymers is introduced.
Takehiro Hirao and Takeharu Haino, Chem. Asian. J., 2022, e202200344.
●Chirality Induction in a Hydrophilic Metallohelicate
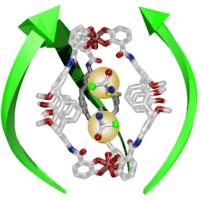
The title compound is a water-soluble triply stranded metallohelicate 1Fe composed of TEG-chain-installed calix[4]arene-based bis-bidentate ligands and Fe3+ cations. This compound can incorporate up to two molecules of small chiral cations (G1 and G2). Interestingly, G1 shows positive cooperativity for host-guest complexation, whereas G2 exhibits noncooperativity, despite having a greater affinity for 1Fe than G1. Density functional theory (DFT) calculations indicate the metallohelicate cavity expands upon encapsulation of the first guest. This conformational change facilitates the entrapment of the second guest and explains why the interaction parameters of G1 and G2 are larger than 1, despite electrostatic repulsion between the guests. The DFT calculation predicts an intermolecular interaction between the phenyl groups of G1 in the cavity, whereas no such interaction is found for G2. This difference can explain the distinct molecular recognition of G1 and G2. CD spectroscopy shows that both (R)-G1 and (R)-G2 induce (M)-helicity in 1Fe and vice versa. DFT calculations suggest that the chirality of the guests is transmitted to 1Fe via CH/O hydrogen bonds between the guest and the C=O bond on the calix[4]arene strand. The CD intensity of 1Fe is nonlinear in the ee% of (R)- and (S)-G1, indicating that the presence of the first guest increases the affinity of 1Fe toward a second guest with the same chirality due to preorganization by the first guest.
Masayuki Morie, Ryo Sekiya, and Takeharu Haino, Chem. Asian J., 2022, 17, e202200275.
●Host–Guest Complexation of Bisporphyrin Cleft and Electron-Deficient Aromatic Guests

The host–guest complexation of a bisporphyrin cleft with various electron-deficient guest molecules was studied in solution and in the solid-state. X-ray crystal structures of a bisporphyrin cleft with naphthalene dianhydride and 2,4,7-trinitrofluorenone reveal that these guest molecules were located within the bisporphyrin cleft and formed ideal π–π stacking interactions in a host–guest ratio of 1:1. Isothermal titration calorimetry determined the binding constants and thermodynamic parameters for the 1:1 host–guest complexations in 1,2-dichloroethane and toluene. Two types of enthalpy–entropy compensation effects were found: (1) The tightly stacked host–guest structures restrict guest movement within the cleft, which results in significant desolvation with large intrinsic entropies. (2) The loosely bound guests maintain their molecular freedom within the bisporphyrin cleft, which leads to less desolvation with small intrinsic entropies. Chiral guest encapsulation directed the clockwise and anticlockwise twisted conformations of the bisporphyrin units, which induced bisignate CDs.
Naoyuki Hisano and Takeharu Haino, J. Org. Chem., 2022, 87, 4001-4009.
●Chiral Supramolecular Polymer Formed via Host-Guest Complexation of an Octaphosphonate Biscavitand and a Chiral Diammonium Guest
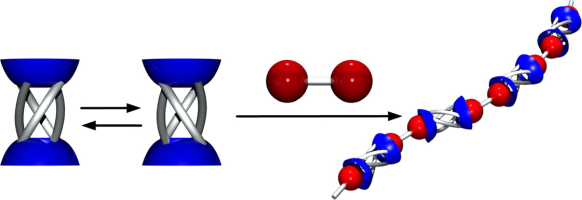
Chiral supramolecular polymers were constructed through the host-guest complexation of an octaphosphonate biscavitand and a chiral diammonium guest. Isothermal titration calorimetry determined that host-guest complexation was enthalpy- and entropy-favored with the high binding constants. Diffusion-ordered NMR spectroscopy and viscometry of the host-guest solution revealed that supramolecular polymerization occurred, which most likely followed a ring-chain mechanism. The cyclic oligomers and the supramolecular polymer chains were visualized by atomic force microscopy. Circular dichroism was observed when the octaphosphonate biscavitand and the chiral diammonium guest were mixed, which suggested that chirally twisted supramolecular polymers were formed. A chiral supramolecular polymer was constructed through host-guest complexation between an octaphosphonate biscavitand and a chiral diammonium guest. A ring-chain mechanism participated in supramolecular polymerization, where a certain amount of cyclic oligomers most likely competed with supramolecular polymers. The cyclic oligomers and the supramolecular polymer chains were visualized by atomic force microscopy. The stereogenic centers of the guest chirally twisted the supramolecular polymer chains in solution.
Koki Hamada, Daisuke Shimoyama, Takehiro Hirao, Takeharu Haino, Bull. Chem. Soc. Jpn., 2022, 95, 621-627.
●Electrochromism of Nanographenes in the Near-Infrared Region

Nanographene (NG) is a potential candidate for organic EC materials because of its large π-conjugated system, chemical stability, absorption band covering the visible region, and tunable optical properties by postsynthetic modification. We show that NGs carrying redox-active triphenylamine (TPA) units covalently linked to the NG edge function as EC materials in the NIR region. The hybrid materials can be obtained by the installation of TPA units onto the NG edge and display changes in the absorption spectrum in the NIR region extending to a wavelength of over 2000 nm upon one-electron oxidation and reduction at low potentials (<1.1 V). Time-dependent unrestricted density functional theory calculation of a model NG at the UB3LYP/6-31G(d,p) level of theory suggests that a narrow energy gap between the basal plane and the oxidized TPA unit is responsible for the observed EC function in the NIR region.
Ikuya Matsumoto, Ryo Sekiya, Hiroji Fukui, Ren-De Sun, and Takeharu Haino, Angew. Chem. Int. Ed., 2022, 61, e202200291.(Hot Paper, Back Cover)
●Non-Racemically Twisted Supramolecular Fullerene Polymers
平尾岳大(Hirao Takehiro)・灰野岳晴(Haino Takeharu)
高分子, 71巻, 1号, pp. 6-6, 2022
●Nanographene – A Scaffold of Two-Dimensional Materials
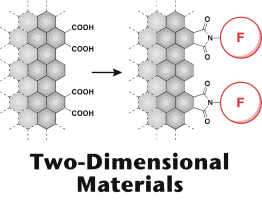
Substances can be divided into 0D to 3D species based on the number of repeating units (atom, ion, and molecule) and their arrangements in space (point, linear, layer, and solid). Discrete substances belong to 0D species, polymers are examples of 1D species, and molecular crystals are 3D species. Most of the substances belong to one of these species. On the other hand, those categorized into 2D species wherein the repeating units organize a layer are less explored. 2D species have a surface and edges. The incorporation of these structural features into a molecular design can realize multifunctionalized systems that are difficult to achieve by conventional organic synthesis. The development of 2D species is, therefore, the frontier of organic, inorganic, and polymer chemistry. Nanographenes (NGs) are suitable scaffolds for realizing 2D species due to several factors, such as chemical stability and oxygen-containing functional groups on the surface and on the edge, allowing postsynthetic modifications. Our group has utilized NGs with tens of nanometers in diameters for developing 2D species. Carboxy groups on the edge enable us to install various substituents into NGs, offering NG-based functional materials. These studies demonstrate that the integration of NGs with organic chemistry can widen the scope of their applications other than optical materials that are a main application of NGs. We introduce our recent studies on the development of NG-based functional materials realized by postsynthetic modifications. We hope that this account will contribute to the development of the chemistry of 2D species.
Ryo Sekiya and Takeharu Haino, Chem. Rec., 2022, 22, e202100257.(Cover Picture)
●Resorcinarene-based Supramolecular Capsules – Supramolecular Functions and Applications
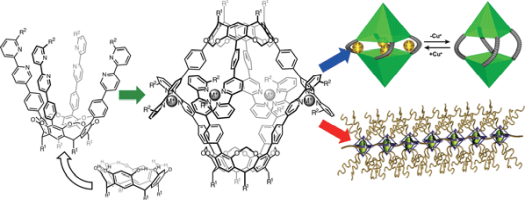
A resorcinarene is a synthetic macrocycle comprising four resorcinol molecules covalently linked by methylene bridges. The interannular bridges produce a cavitand, which possesses a bowl-shaped structure. We have developed supramolecular capsules formed through Ag(I) and Cu(I) coordination-driven self-assembly of cavitands possessing 2,2’-bipyridyl arms at the upper rim. The self-assembled capsules accommodate various molecular guests and supramolecular assemblies possessing acetoxy groups. The host-guest chemistry of the molecular capsules is applied to fabricate supramolecular polymers. This account describes the recent developments in supramolecular chemistry of resorcinarene-based coordination capsules and describes the brief history of resorcinarene-based capsules and related capsules.
Ryo Sekiya, Kentaro Harada, Natsumi Nitta, and Takeharu Haino, Synlett, 2022, 33, 518-530.
2021
●Self-Sorting Behavior in Supramolecular Fullerene Polymerization Directed by Host-Guest Complexation between Calix[5]arene and C60
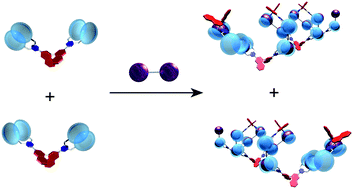
We describe self-sorting supramolecular polymerization that uses chiral calix[5]arene hosts and a dumbbell-shaped fullerene guest. In a solution containing the racemic host and the guest, the (S)-host and the (R)-host preferably formed their homomeric complexes to form helical supramolecular fullerene polymers in a self-sorting manner. The self-sorting behavior has been studied using diffusion-ordered 1H NMR (DOSY) and circular dichroism (CD) studies. The present findings show that it is possible to accomplish controlled supramolecular polymerization.
Takehiro Hirao, Naoka Fujii, Yoshiki Iwabe, and Takeharu Haino,Chemm.Commun., 2021, 57, 11831-11834.
●Synthesis and Conformational Characteristics of Calix[4]arene Oligomers
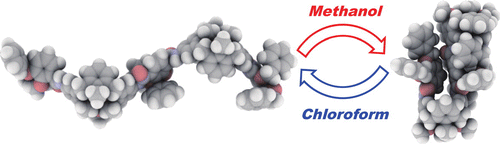
Lower- and/or upper-rim functionalization of calix[4]arene realized a variety of calix[4]arene systems. Compared to these monomeric calix[4]arenes, covalently linked calix[4]arene oligomers have not been studied well. Calix[4]arene oligomers can be utilized as building blocks of supramolecular complexes as well as for the synthesis of calix[4]arene polymers. This background motivated us to develop synthetic procedures for calix[4]arene oligomers and to conduct conformational analysis of these oligomers. We produce oligomers ranging from the monomer to the pentamer. The coupling of the pentamer with 2,3-dibenzyloxy-1,4-benzenedicarboxylic acid can access a decamer and oligomers. NMR measurements, X-ray crystal structure analysis, and computational studies demonstrate that calix[4]arene oligomers can regulate their length by changing the conformations of the calix[4]arene cores.
Masauki Morie, Ryo Sekiya, and Takeharu Haino, Bull. Chem. Soc. Jpn, 2021, 94, 2792-2799.(Selected Paper)
●Gas-Phase UV Spectroscopy of Chemical Intermediates Produced in Solution: Oxidation Reactions of Phenylhydrazines by DDQ
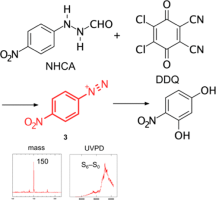
In this study, we demonstrated cold gas-phase spectroscopy of chemical intermediates produced in solution. Herein, we combined an electrospray ion source with a T-shaped solution mixer for introducing chemical intermediates in solution into the gas phase. Specifically, the oxidation reaction of 2-(4-nitrophenyl)hydrazinecarboxaldehyde (NHCA) by 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) was initiated by mixing the methanol solutions of NHCA and DDQ in the T-shaped mixer, and the chemical species were injected into the vacuum apparatus for ultraviolet photodissociation (UVPD) spectroscopy. A cationic intermediate was strongly observed at m/z 150 in the mass spectrum, and the UVPD spectrum was observed under cold (∼10 K) gas-phase conditions. The UVPD spectrum showed a strong, broad absorption at ∼38,000 cm–1, accompanied by a relatively weak component at ∼34,000 cm–1. These spectral patterns can be ascribed to a diazonium cation intermediate, whose existence has been predicted in a previous study. This report indicates that cold gas-phase UV spectroscopy can be a useful method for identifying the structure of chemical intermediates produced in solution.
Shiori Machida, Motoki Kida, Satoru Muramatsu, Takehiro Hirao, Takeharu Haino, and Yoshiya Inokuchi, J. Phys. Chem. A, 2021, 125, 6697-6702.
●Solvent-Directed Formation of Helically Twisted Stacking Constructs via Self-Assembly of Tris(phenylisoxazolyl)benzene Dimers
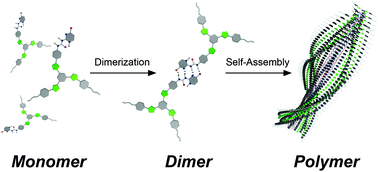
Ureido-pyrimidinone (UPy)-appended tris(phenylisoxazolyl)benzenes were synthesized. The UPy moieties of the tris(phenylisoxazolyl)benzenes stably formed self-complementary dimers in solution. The dimers self-assembled to form helically twisted stacking constructs driven by π-π stacking interactions of UPy dimer moieties and dipole-dipole interactions of isoxazole units. Strong association affinity was seen within the stacking constructs compared with the previously reported isoxazole derivatives owing to the auxiliary π-π stacking interaction. Notably, tris(phenylisoxazolyl)benzenes showed an environmentally responsive nature. The absorption band, emission intensity, and size of ensembles significantly depended on the mixing ratio of CHCl3 and methylcyclohexazne (MCH). Also, a sharp on-off switching nature was seen in their circular dichroism (CD) and circularly polarized luminescence (CPL) spectra in response to the mixing ratio of CHCl3 and MCH. CD and CPL were activated only at a certain mixing ratio of CHCl3/MCH, thus showing potential for the creation of molecular sensors.
Yudai Ono, Takehiro Hirao and Takeharu Haino , Org. Biomol. Chem., 2021, 19, 7165-7171.
●Self-Complementary Structure of Bisporphyrin Dimer

The crystal structure of a bisporphyrin cleft molecule revealed that the head-to-head dimeric structure is directed by the intermolecular self-complementary hydrogen-bonding interactions of amide groups and π-π stacking interactions. The UV/Vis absorption spectrum of the homodimeric structure in the solid-state resulted in a broad Soret band. Time-dependent density functional theory (TD-DFT) calculation of the dimer indicated that an intra- and intermolecular charge transfer transition as well as a π-π* transition were responsible for the observed broad Soret bandThe crystal structure of a self-complementary bisporphyrin dimer is reported. The head-to-head dimeric structure was maintained by the assistance of hydrogen bonding interactions and π-π stacking interactions. The UV-Vis absorption spectrum of the dimer showed a broad Soret band in the solid state. TD-DFT calculation of the dimeric structure revealed that a local π-π* electronic transition and intra- and intermolecular charge transfer electronic transition most likely contribute to band broadening at the Soret band.
Naoyuki Hisano ,Takehiro Hirao , and Takeharu Haino, Chem. Lett., 2021, 50, 1844-1847.
●Negative Coorperativity in Guest Binding of Ditopic Self-Folding Biscavitand

A brand-new self-folding biscavitand was synthesized from a feet-to-feet-connected bisresorcinarene. The X-ray crystal structure of the biscaivtand showed that the two cavities are tightly connected with four butylene linkages. The conformationally coupled two cavities accommodated two cationic guests, showing a homotropic negative cooperativity in nonpolar solvents (toluene and chloroform). A polar tetrahydrofuran solvent weakened the cyclic hydrogen bonding interactions of the biscavitand, which resulted in noncooperative guest binding.
Haruna Fujimoto, Daisuke Shimoyama, Katsuo Katayanagi, Naomi Kawata, Takehiro Hirao, and Takeharu Haino, Org. Lett., 2021, 23, 16, 6217–6221.(Supplementary Journal Cover)
DOI:10.1021/acs.orglett.1c01837
●SUPRAMOLECULAR CHEMISTRY OF FULLERENES (Book Chapter)
Takehiro Hirao and Takeharu Haino, Handbook of Fullerene Science and Technology
●ナノグラフェンの有機合成化学による構造修飾と機能発現, Chemical Modification of Nanographenes and Their Functions
Ryo Sekiya and Takeharu Haino, Journal of Synthetic Organic Chemistry, Japan(有機合成化学協会誌), 2021, 79, 743-753.
DOI:10.5059/yukigoseikyokaishi.79.743
●(ナノ)グラフェン
(解説)有機合成化学協会誌2021年8月号, p792
●トップダウン法により得られるナノグラフェンの有機化学
(解説)特集:ナノカーボン材料はどこまで進んだか?
関谷亮(Sekiya Ryo)・灰野岳晴(Haino Takeharu),化学と工業, 2021年6月号,p409-411.
●Polymerization of a Biscalix[5]arene Derivative
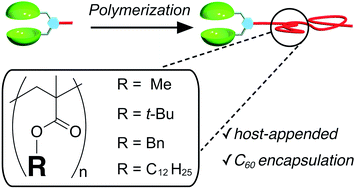
Recent decades have seen an increased interest in the preparation of polymers possessing host or guest moieties as the end group, which has enabled new polymeric materials such as self-healable, shape-memory, and stimuli-responsive materials. Such polymers are commonly synthesized by tethering the host or guest moieties to polymers. On the other hand, there are limited reports demonstrating the preparation of host- or guest-appended polymers by directly polymerizing the corresponding host- or guest-appended monomers, which is valuable for easy access to diverse polymers from single molecular species. However, reactive host and/or guest moieties of the monomer interfere with the polymerization reaction. Here, we report that a biscalix[5]arene host-appended molecule can be polymerized with various monomers to form the corresponding host-appended polymers. The host-guest complexation behavior of calix[5]arene-appended polymers with fullerene derivatives was studied by 1H NMR and UV/vis spectroscopic techniques, which revealed that the long polymer chains did not prevent host-guest complexation even when the fullerene derivative was equipped with a polymer chain. Thus, the present study shows the potential for developing polymers that have various combinations of polymer chains.
Takehiro Hirao, Kazushi Fukuta, and Takeharu Haino, RSC Adv., 2021, 11, 17587-17594.
●Self-assembly of neutral platinum complexes possessing chiral hydrophilic TEG chains
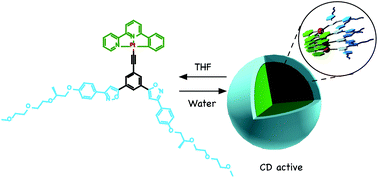
Neutral platinum complexes that possess chiral triethylene glycol (TEG) moieties were synthesized. The platinum complexes formed helically twisted stacked assemblies in chloroform and toluene, which were studied by 1H NMR, UV/vis spectroscopy, and emission spectroscopy. On the other hand, emissive micellar aggregates were observed in a THF/water mixed solvent. Dynamic light scattering (DLS) experiments revealed that micellar aggregates with a diameter (d) of ≈100 nm emitted strong light, whereas the monomeric form and large aggregates (d > 500 nm) did not show luminescence efficiently. Furthermore, the micellar aggregates were twisted chirally, where the twisted direction was determined by the chirality of the TEG moieties. The assemblies were observed to be solvent responsive, which allows for the modulation of the nanostructure by changing the solvent polarity.
Masaya Yoshida, Takehiro Hirao, and Takeharu Haino, Org. Biomol. Chem., 2021, 19, 5303-5311.(Cover Picture)
●Self-Assembling Behavior and Chiroptical Properties of Carbazole-Cored Phenyl Isoxazolyl Benzenes
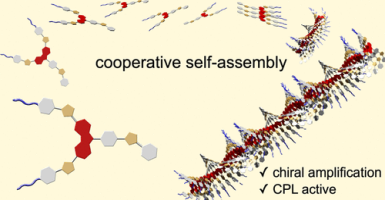
Carbazole-cored phenyl isoxazolyl benzenes possessing chiral moieties were synthesized. The molecules self-assembled to form stacked supramolecular assemblies in an isodesmic fashion in chloroform, whereas the molecules preferably assembled in a cooperative fashion in methylcyclohexane (MCH), which was determined by spectroscopic methods, including UV–vis absorption, fluorescence, and 1H NMR spectroscopy. Clear nucleation and elongation processes were observed in the plot of the degree of aggregation (αagg) against temperature, which allowed us to determine the elongation temperature (Te), the enthalpic gain in the elongation process (ΔHe), the equilibrium constant between nucleation and elongation (Ka), and the degree of polymerization at the elongation temperature ([Nn(Te)]). Circular dichroism (CD) and circularly polarized luminescence (CPL) studies revealed the formation of helically stacked assemblies in solution. Moreover, the majority-rule effect was clearly observed in the solutions of mixtures of (S)- and (R)-1, indicating the chiral amplification behavior of the helically stacked assemblies consisting of (S)- and (R)-1. AFM provided morphological insight into the assemblies on mica, which clearly indicates the formation of polymeric assemblies in the solid state.
Yudai Ono, Takehiro Hirao, Toshiaki Ikeda, and Takeharu Haino, J. Org. Chem., 2021, 86, 5499-5505.(Supplementary Journal Cover)
●Self-Assembly of Nanographenes
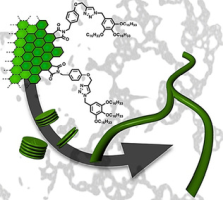
Suitably decorated small aromatic systems can organize stacked structures that display interesting properties arising from their unique morphologies. Although nanographenes produced by top-down methods have graphitic domains and can in principle be applied for such supramolecular systems, to the best of our knowledge, no such example has been reported thus far. This is partly because of their limited solubility in organic solvents and partly because of their wide lateral size distribution. To realize nanographene-based supramolecular aggregates, nanographenes carrying alkyl chains with narrow lateral size distributions are employed. We find that the nanographenes undergo self-assembly and that self-assembly is regulated by concentration, solvent polarity, temperature, and sonication. Optical measurements and AFM images indicate that stacked structures are possible candidates for aggregates. A molecular mechanics calculation suggests that graphitic domains inside the nanographenes can contact those of the nearest neighbors in the aggregates, while graphitic domains near the edge are difficult to make contact due to steric congestion. This model can rationalize a unique PL response to temperature. The nanographenes showed concentration-dependent morphologies on mica, stacked structures at low concentrations and polymer-like network structures on mica at higher concentrations.
Ikuya Matsumoto, Ryo Sekiya, and Takeharu Haino, Angew. Chem. Int. Ed., 2021, 60, 12706-12711.(Hot Paper) (Frontispiece)
●Helically Organized Fullerene Array in a Supramolecular Polymer Main Chain

To date, supramolecular chemistry techniques have been applied to fullerene polymer synthesis, enabling the development of main-chain fullerene polymers whose primary structure is well regulated, including linear, dendritic, and net-like fullerene arrays. These research achievements have led to an intriguing scientific challenge to create main-chain fullerene polymers with higher structural regulation. Here, we report the fabrication of a helically organized fullerene array based on the supramolecular polymerization of chiral ditopic tetrakiscalix[5]arene hosts and a dumbbell-shaped fullerene. The molecular association between the chiral hosts and the dumbbell-shaped fullerene resulted in sizable supramolecular polymers in solution, with the highest degree of polymerization of more than 32. The achiral dumbbell-shaped fullerene exhibited circular dichroism in the π-π* transition bands arising from the fullerene moieties through supramolecular polymerization. End-capping experiments of the supramolecular helical polymers showed that the chirally twisted conformation of the dumbbell-shaped fullerene was directed by supramolecular polymerization. Finally, the helical morphology of the supramolecular polymer chain was visualized by atomic force microscopy. The successful development of helical main-chain fullerene polymers would break new ground in fullerene chemistry.
Tekehiro Hirao, Yoshiki Iwabe, Naoka Fujii, Takeharu Haino, J. Am. Chem. Soc., 2021, 143, 4339-4345.
●Folding and Unfolding of Acetoxy Group-Terminated Alkyl Chains Inside a Size Regulable Hemicarcerand

A resorcinarene-based hemi-carcerand, which consists of two cavitands covalently linked to each other by four alkyl chains, allows structural expansion and contraction by demetalation and metalation of Cu(I) cations with a size change of approx-imately 12 Å. This metal-mediated switching of the two states regulates the conformations of acetoxy group-terminated alkyl chains. A guest binding study reveals the encapsulation of heptyl to undecyl chains in metal-free and Cu(I)-coordinated capsules. The chemical shifts of the acetoxy groups of the bound guests are the same in the metal-free capsule, while those in the Cu(I)-coordinated one differ from each other. This indicates that the metal-free capsule regulates its size to the bound guests, while the bound guests adopt their conformations to the cavity of the Cu(I)-coordinated capsules. 1H NMR measurements and molecular mechanics calculations suggest that the bound guests have extended conformations in the metal-free capsule, while the Cu(I)-coordinated capsule forces the bound guests to adopt folded conformations. The presence of folded conformations is supported by the conformational study with structurally similar capsules and a non-symmetric guest, allowing us to observe NOEs stemming from folded conformations of the guest in the cavity.
Kentaro Harada, Ryo Sekiya, and Takeharu Haino, J. Org. Chem., 2021, 86, 4440-4447.(Supplementary Journal Cover)
●Translational Isomers of N-sulfonylated [3]Catenane: Synthesis and Isomerization
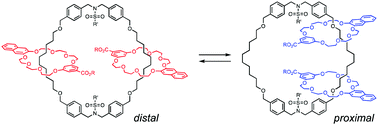
N-Sulfonylated [3]catenanes, which exist as two translational isomers, were synthesized. The X-ray crystal structure of the distal isomer of [3]catenane, which has higher symmetry, revealed hydrogen bonds involving the carboxylic acid moieties on the terminal rings. The thermodynamic parameters of the isomerization revealed that this hydrogen bonding influenced the isomerization process.
Hajime Iwamoto, Yuki Ishizu, Eietsu Hasegawa, Ryo Sekiya and Takeharu Haino , Chem. Commun., 2021, 57, 1915-1918.
●Nanographenes from Distinct Carbon Sources
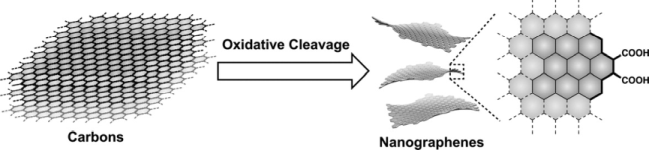
This article reports the production protocol of nanographenes and the effect of the reaction conditions on their structures and optical properties. These fundamental studies are of value for exploring suitable reaction conditions for the production of nanographenes with desirable properties. Graphite, finely crushed graphite powders, and artificial graphite, all of which are commercially available, are employed. Nanographenes are produced by the acid-assisted oxidative cleavage of the parent carbons followed by neutralization and deionization. The use of dialysis membranes for the size separation of nanographenes offers nanographenes with a specific size distribution, thereby allowing their structures and optical properties to be compared. Experiments demonstrate that small amounts of acids (60 ml of conc. H2SO4 and 20 mL of 60% HNO3) and oxidation for 12 h promotes a more efficient and cost-effective production of nanographenes from 2 g of a carbon source. The functionalization of the nanographene edges with p-propargyloxybenzyl amine confirms that the armchair edge with two carboxy groups is the dominant edge structure, irrespective of the carbon source.
Ikuya Matsumoto, Ryo Sekiya, and Takeharu Haino, Bull. Chem. Soc., Jpn., 2021, 94, 1394-1399.(Selected Paper) (Inside Cover)
●Blueish-white-light-emitting Nanographenes Developed by Pd-Catalyzed Suzuki-Miyaura Cross Coupling Reactions
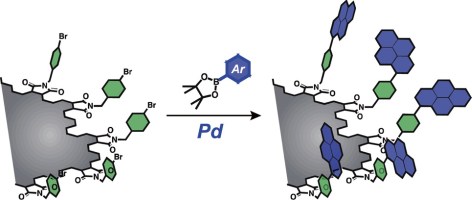
Top-down methods produce nanographenes with many carboxy groups on their edges. These functional groups can be utilized for developing multichromophoric systems. As proof of concept, pyrene is installed on the edges by Pd-catalyzed cross-coupling reactions. The lack of monomer emissions from the functionalized nanographenes indicates that the neighboring chromophores are sufficiently distant to form the excimer. The pyrene-installed nanographene emits bluish-white-light. Its lipophilic nature allows fabricating a nanographene-dispersed polymethyl methacrylate film emitting visible light.
Shohei Nishitani, Ryo Sekiya, Ikuya Matsumoto, and Takeharu Haino, Chem. Lett., 2021, 50, 664-667.
●Programmed Dynamic Covalent Chemistry System of Addition-Condensation Reaction of Phenols and Aldehydes

Application of a dynamic covalent chemistry strategy to the reversible reaction system of resorcinol and α, ω-alkanedials [(CH2)m(CHO)2] (m = 2 ~ 10) in ethanol in the presence of hydrogen chloride (HCl) solution as a catalyst at 80 °C for 48 h afforded the thermodynamically most stable products with high selectivity. The reaction of 1,4-butanedial afforded a ladder polymer containing calixarene skeletons in the main chain in quantitative yield. 1,5-Pentanedial gave Noria, a water-wheel-like cyclic oligomer, in high yield. Calixarene-dimer-type cyclic oligomers were formed selectively from 1,6-hexanedial, 1,8-octanedial, 1,10-decanedial, and 1,12-dodecanedial, while calixarene-trimer-type cyclic oligomers were obtained selectively from 1,7-heptanedial, 1,9-nonanedial, and 1,11-undecanedial. The Noria like macrocyles NoriaPY NoriaMP and NoriaEP could be also synthesized via DCC system using pyrogallol, 3-methoxyphenol, and 3-ethoxyphenol. A triple-ringed[14]arene could be synthesized via DCC system using the reaction of 2-methylresorcinol and m-benzenedicarbaldehyde.
Hiroto Kudo, Daisuke Shimoyama, Ryo Sekiya, and Takeharu Haino, Chem. Lett., 2021, 50, 825-831.
●Supramolecular fluorescent sensors: An historical overview and update

Since as early as 1867, molecular sensors have been recognized as being intelligent “devices” capable of addressing a variety of issues related to our environment and health (e.g., the detection of toxic pollutants or disease-related biomarkers). In this review, we focus on fluorescence-based sensors that incorporate supramolecular chemistry to achieve a desired sensing outcome. The goal is to provide an illustrative overview, rather than a comprehensive listing of all that has been done in the field. We will thus summarize early work devoted to the development of supramolecular fluorescent sensors and provide an update on recent advances in the area (mostly from 2018 onward). A particular emphasis will be placed on design strategies that may be exploited for analyte sensing and corresponding molecular platforms. Supramolecular approaches considered include, inter alia, binding-based sensing (BBS) and indicator displacement assays (IDAs). Because it has traditionally received less treatment, many of the illustrative examples chosen will involve anion sensing. Finally, this review will also include our perspectives on the future directions of the field.
Chenxing Guo, Adam C. Sedgwick, Takehiro Hirao, and Jonathan L. Sessler, Coord. Chem. Rev., 2021, 427, 213560.
●A Light‐Harvesting/Charge‐Separation Model with Energy Gradient Made of Assemblies of meta‐pyridyl Zinc Porphyrins

Self‐assembly of porphyrins is a fascinating topic not only for mimicking chlorophyll assemblies in photosynthetic organisms but also for the potential of creating molecular‐level devices. Herein we have prepared zinc porphyrin derivatives bearing a meta‐pyridyl group at the meso‐position and studied their assemblies in chloroform. Among porphyrins studied, one with a carbamoylpyridyl moiety (ZnPPyC) gave distinct 1H NMR spectrum in CDCl3, which allowed us to probe the supramolecular structure in solution in detail. Ring‐current induced chemical shift changes in 1H NMR, together with vapor pressure osmometry and diffusion‐ordered NMR spectroscopy, among other evidence, suggested that the porphyrin molecules form a trimer in a triangular cone structure. Further incorporation of a directly‐linked porphyrin‐ferrocene dyads with the same assembling properties in the assemblies lead to a rare light‐harvesting/charge‐separation model, in which an energy gradient is incorporated and reductive quenching occurs.
Joe Otsuki, Takumi Okumura, Kosuke Sugawa, Shin-ichiro Kawano, Kentaro Tanaka, Takehiro Hirao, Takeharu Haino, Yu Jin Lee, Seongsoo Kang, Dongho Kim, Chem. Eur. J. 2021, 27, 4053-4063.
●Stereoselectivity in Dehydrative Cyclic Trimerization of Substituted 4-Alkylaminobenzoic Acids

Cyclic trimerization of substituted 4-alkylaminobenzoic acids were investigated. From NMR analyses of DiMeO_C3A with two methoxy groups, which was obtained by using SiCl4 as a dehydrative condensation reagent and purified by preparative GPC, exhibited the syn/anti ratio of 60/40. On the other hand, 3Br_C3A with one bromine group at the ortho-position relative to the amide nitrogen was synthesized by using PPh3/Cl3CCCl3 as a dehydrative condensation reagent and isolated by SiO2 colum chromatography. 3Br_C3A showed an inverse stereoselectivity, namely, the syn/anti ratio of 25/75 was calculated based on the conprehensive NMR analyses. The population of stereoisomers had no relationship with the dehydrative condensation reagent and reaction temperature. Solvent character also had a negligible influence on the syn/anti ratio in solution reflecting the rigid structure of 3Br_C3A.
Koji Takagi, Hinako Yamaguchi, Daiki Miyamoto, Yuka Deguchi, Takehiro Hirao and Takeharu Haino, New J. Chem., 2021, 45, 1187-1193.
●Calix[4]arene-Based Triple-Stranded Metallohelicate in Water

The title complex is a triple-stranded metallohelicate organized by the self-assembly of 5,17-difunctionalized calix[4]arenes and metal cations with octahedral coordination geometry. Due to hydrophilic triethylene glycol chains on the lower rim of the calix[4]arene, the metallohelicate can encapsulate cationic guests in water. NMR and UV-vis titration experiments reveal that the metallohelicate captures a pyridinium guest with an alanine derivative to form a host-guest complex with a host-guest ratio of 1:1. CD spectroscopy confirms the bias of the P- and M-helical sense of the metallohelicate by the captured guest. The metallohelicate captures two molecules of dicationic N,N’-dimethyl-DABCO and monocationic N-methyl quinuclidine, exhibiting a positive allosteric effect. 1H NMR titration experiments indicate that the bound guests are in close proximately to the aromatic rings of the ligands. Molecular mechanics calculations based on the UV-vis and NMR observations suggest that the first guest preorganizes the conformation of the metallohelicate to facilitate access of the second guest to the cavity.
Masayuki Morie, Ryo Sekiya, and Takeharu Haino, Chem. Asian J., 2021, 16, 49-55.(Cover Picture)
● Edge Functionalized Nanographenes

Nanographenes (NGs) have recently emerged as new carbon materials. Their nanoscale size results in a size-dependent quantum confinement effect, opening the band gap by a few eV. This energy gap allows NGs to be applied as optical materials. This property has attracted researchers across multiple scientific fields. The photophysical properties of NGs can be manipulated by introducing organic groups onto their basal planes and/or into their edges. In addition, the integration of organic functional groups into NGs results in NG-based hybrid materials. These features make the postsynthetic modification of NGs an active research area. Since obtainable information on chemically functionalized NGs is limited due to their nonstoichiometry and structural uncertainty, their structural characterization requires a combination of multiple spectroscopic methods. Therefore, information on the characterization procedures of recently published chemically functionalized NGs is of value for advancing the field of NG-based hybrid materials. The present review focuses on the structural characterization of chemically functionalized NGs. We hope this review will help to advance this field.
Ryo Sekiya and Takeharu Haino, Chem. Eur. J., 2021, 27, 187-199. (This article was selected as a Review Showcase.)
2020
●Conformation of K+(Crown Ether) Complexes Revealed by Ion Mobility-Mass Spectrometry and Ultraviolet Spectroscopy

The conformation and electronic structure of dibenzo-24-crown-8 (DB24C8) complexes with K+ ion were examined by ion mobility–mass spectrometry (IM–MS), ultraviolet (UV) photodissociation (UVPD) spectroscopy in the gas phase, and fluorescence spectroscopy in solution. Three structural isomers of DB24C8 (SymDB24C8, Asym1DB24C8, and Asym2DB24C8) in which the relative positions of the two benzene rings were different from each other were investigated. The IM–MS results at 86 K revealed a clear separation of two sets of conformers for the K+(SymDB24C8) and K+(Asym1DB24C8) complexes whereas the K+(Asym2DB24C8) complex revealed only one set. The two sets of conformers were attributed to the open and closed forms in which the benzene–benzene distances in the complexes were long (>6 Å) and short (<6 Å), respectively. IM–MS at 300 K could not separate the two conformer sets of the K+(SymDB24C8) complex because the interconversion between the open and closed conformations occurred at 300 K and not at 86 K. The crown cavity of DB24C8 was wrapped around the K+ ion in the complex, although the IM–MS results availed direct evidence of rapid cavity deformation and the reconstruction of stable conformers at 300 K. The UVPD spectra of the K+(SymDB24C8) and K+(Asym1DB24C8) complexes at ∼10 K displayed broad features that were accompanied by a few sharp vibronic bands, which were attributable to the coexistence of multiple conformers. The fluorescence spectra obtained in a methanol solution suggested that the intramolecular excimer was formed only in K+(SymDB24C8) among the three complexes because only SymDB24C8 could possibly assume a parallel configuration between the two benzene rings upon K+ encapsulation. The encapsulation methods for K+ ion (the “wraparound” arrangement) are similar in the three structural isomers of DB24C8, although the difference in the relative positions of the two benzene rings affected the overall cross-section. This study demonstrated that temperature-controlled IM–MS coupled with the introduction of appropriate bulky groups, such as aromatic rings to host molecules, could reveal the dynamic aspects of encapsulation in host–guest systems.
Sota Tanaka, Yomoyuki Ujihira, Mayuko Kubo, Motoki Kida, Daisuke Shimoyama, Satoru Muramatsu, Manabu Abe, Takeharu Haino, Takayuki Ebata, Fuminori Misaizu, Keijiro Ohshimo, and Yoshiya Inokuchi, J. Phys. Chem. A, 2020, 124, 48, 9980–9990.
●Absorption of Chemicals in Amorphous Trisresorcinarene
Trisresorcinarene is an interesting class of macrocyclic hosts. Its insoluble nature and conformationally flexible structure allow its application as a solid absorbent. Various aromatic and aliphatic hydrocarbons are absorbed into the amorphous solid of the trisresorcinarene, demonstrating the versatile absorption capability of trisresorcinarene.
Daisuke Shimoyama, Ryo Sekiya, and Takeharu Haino, Chem. Commun., 2020, 56, 12582-12585.
●Feet‐to‐Feet‐Connected Multitopic Resorcinarene Macrocycles

The past three decades have witnessed extraordinary advances in the preparation of macrocycles from a resorcinarene platform. Bridging two or more resorcinarene units through multipoint connections produces multitopic resorcinarene‐based hosts. The unique properties arising from multivalency offer diverse applications, such as molecular flasks, molecular machines, and supramolecular polymers. The face‐to‐face connection of two or more resorcinarenes results in a convergent, expanded guest binding space in which many large guests can be accommodated. In contrast, the feet‐to‐feet connection provides an extroverted, ditopic feature that can lead to different applications. Herein, we highlight the synthesis of extroverted, multitopic resorcinarene macrocycles and discuss their fascinating functions, such as molecular recognition, allosteric guest binding, and supramolecular polymerization.
Daisuke Shimoyama and Takeharu Haino, Asian. J. Org. Chem., 2020, 1718-1725.
●灰野岳晴教授のプロフィールがAngew. Chem. Int. Ed.に掲載されました。
●One-Dimensional Arrangement of NORIA in the Solid-state

NORIA is a synthetic macrocycle consisting of twelve resorcinol rings. The hydroxy groups surrounding the surface of NORIA make it difficult for NORIA to be dissolved in organic solvents, except for polar solvents, a property which is ideal for developing solid-state materials. For developing solid-state materials, information about the effect of the crystallization conditions on the crystal packing of NORIA is required, although only limited examples have been reported so far. We find that the crystal packing of NORIA depends heavily on the crystallization conditions. Single crystals are grown in DMF solutions by the slow diffusion of pentane, cyclohexane, methylcyclohexane, benzene, and toluene into the solution. X-ray diffraction analysis demonstrates that the saturated hydrocarbon vapors induce the molecules to organize into a triclinic crystal packing, whereas another crystal packing, wherein NORIA is arranged linearly in the hexagonal crystal, is formed in the case of aromatic vapors. In the five crystals, DMF molecules are trapped by NORIA and interact with neighboring hosts through hydrogen bonding, indicating that the DMF molecules function as linkers. The X-ray crystal structures suggest that the planarity of the guests is a factor behind the formation of the hexagonal crystal packing. Electron density maps and the solvent accessible surface of NORIA suggest that small molecules, such as water, can access the cavity. The large solvent-accessible volumes in the unit cells of both crystal packings demonstrate that NORIA functions as an excellent building block for lattice inclusion compounds.
Daisuke Shimoyama, Ryo Sekiya, Hiroyuki Maekawa, Hiroto Kudo, and Takeharu Haino, CrystEngComm, 2020, 22 ,4740-4747
●A Dual Redox-Responsive Supramolecular Polymer Driven by Molecular Recognition between Bisporphyrin and Trinitrofluorenone
A dual redox-responsive supramolecular polymer driven by molecular recognition between bisporphyrin (bisPor) and trinitrofluorenone (TNF) has been developed. The supramolecular polymer was degraded into monomers in response to both oxidation and reduction stimuli.
Naoyuki Hisano, Takehiro Hirao, and Takeharu Haino, Chem. Commun., 2020 ,56 ,7553-7556(Cover Picture)
●Construction of Helically Stacked π‐Electron Systems in Poly(quinolylene‐2,3‐methylene) Stabilized by Intramolecular Hydrogen Bonds
π‐Stacked polymers, which consist of layered π‐electron systems in a polymer, can be expected to be used in molecular electronic devices. However, the construction of a stable π‐stacked structure in a polymer is considerably challenging because it requires sophisticated designs and precise synthetic methods. Herein, we present a novel π‐stacked architecture based on poly(quinolylene‐2,3‐methylene) bearing alanine derivatives as the side chain, obtained through the living cyclo‐copolymerization of an o‐allenylaryl isocyanide. In the resulting polymer, the neighboring quinoline rings of the main chain form a layered structure with π–π interactions, which is stabilized by intramolecular hydrogen bonds. The vicinal quinoline units form two independent helices and the whole molecule is a twisted‐tape structure. This structure is established on the basis of UV/CD spectra, theoretical calculations, and atomic‐force microscopy.
Yuki Kataoka, Naoya Kanbayashi, Naoka Fujii, Taka-aki Okamura, Takeharu Haino, and Kiyotaka Onitsuka, Angew. Chem. Int. Ed .,2020 ,59 ,10286-10291
●Self‐Healing Supramolecular Materials Constructed by Copolymerization via Molecular Recognition of Cavitand‐Based Coordination Capsules
The repeating guest units of poly‐(R)‐2 were selectively encapsulated by the self‐assembled capsule poly‐1 possessing eight polymer side chains to form the supramolecular graft polymer (poly‐1 )n⋅poly‐(R)‐2 . The encapsulation of the guest units was confirmed by 1H NMR spectroscopy and the DOSY technique. The hydrodynamic radius of the graft polymer structure was greatly increased upon the complexation of poly‐1 . The supramolecular graft polymer (poly‐1 )n⋅poly‐(R)‐2 was stably formed in the 1:1 host–guest ratio, which increased the glass transition temperature by more than 10 °C compared to that of poly‐1 . AFM visualized that (poly‐1 )n⋅poly‐(R)‐2 formed the networked structure on mica. The (poly‐1 )n⋅poly‐(R)‐2 gelled in 1,1,2,2‐tetrachloroethane, which led to fabrication of distinct viscoelastic materials that demonstrated self‐healing behavior in a tensile test.
Natsumi Nitta, Mei Takatsuka, Shin-ichi Kihara, Takehiro Hirao, and Takeharu Haino, Angew. Chem. Int. Ed., 2020, 59, 16690-16697.
●Helicity of a polyacetylene directed by molecular recognition of biscalixarene and fullerene
Chiral biscalixarenes and a C60-appended polyphenylacetylene have been synthesized. The chiral biscalixarene encapsulated the C60 unit of the phenylpolyacetylene, which induced the preferred helicity of the polyacetylene.
Takehiro Hirao, Yoshiki Iwabe, Hisano Naoyuki, and Takeharu Haino, Chem. Commun., 2020, 56, 6672-6675
●Columnar Organization of Carbo[5]helicene Directed by Peripheral Steric Perturbation
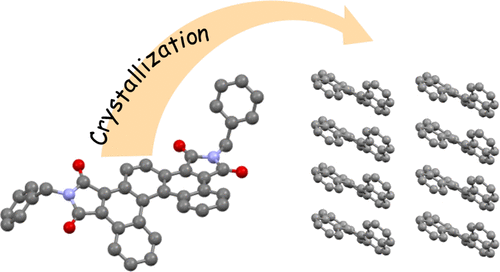
Three carbo[5]helicenes ([5]CHs) containing benzylmaleimide groups displayed columnar stacked organizations, including dislocated, parallel, and alternating (P)- and (M)-helix molecular arrangements in the solid state. The peripheral benzyl groups resulted in very small steric interactions that are responsible for the formation of columnar stacked arrangements in the crystalline state.
Takehiro Hirao, Yudai Ono, Naomi Kawata, and Takeharu Haino, Org. Lett.,2020, 22, 5294-5298(Cover Picture)
DOI:10.1021/acs.orglett.0c01421
●A Supramolecular Approach to Polymer-Shape Transformation via Calixarene-Fullerene Complexation
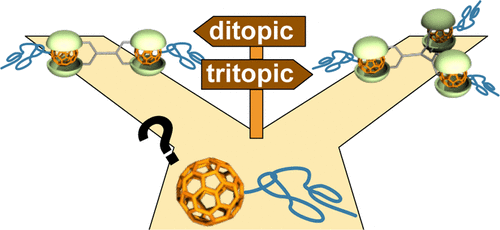
We synthesized fullerene-terminated polymethyl methacrylates (PMMAs), linear ditopic calix[5]arene host, and branched tritopic calix[5]arene host. The calix[5]arene hosts bound to the fullerene moieties of the PMMAs, inducing shape transformation among three different polymer shapes, namely, short PMMA, long PMMA, and star-shaped PMMA, in solution. The transformation was studied using UV/vis spectroscopy, fluorescence spectroscopy, diffusion ordered NMR spectroscopy, size-exclusion chromatography, viscometry, and differential scanning calorimetry. Dynamic light scattering measurements confirmed that the transformation between the individual shapes was induced by application of external stimuli, including prechosen molecules and heating of the solution. Of particular note is that atomic force microscopy revealed that PMMA illustrated an additional shape, namely, a spherical shape, in the solid state due to the cohesive nature of its fullerene moiety. The present study illustrated that a supramolecular approach to polymer-shape regulation allows access to multiple distinct polymer shapes in sequence.
Takehiro Hirao, Kazushi Fukuta, and Takeharu Haino, Macromolecules, 2020, 53, 3563–3570(Cover Picture)
DOI:10.1021/acs.macromol.0c00621
●Chemically Funtionalized Two-Dimensional Carbon Materials

Nanographenes (NGs), also known as graphene quantum dots, have recently been developed as nanoscale graphene fragments. These nanocarbon species can be excited with UV light and emit light from the UV‐to‐visible region. This photoemission has received great attraction across multiple scientific fields. NGs can be produced by cutting off carbon sources or fusing small organic molecules to grow graphitic structures. Furthermore, the organic synthesis of NGs has been intensely studied. Recently, the number of research papers on postsynthetic modifications of NGs has gradually increased. Installed organic groups can tune the properties of NGs and provide new functionalities, opening the door for the development of sophisticated carbon‐based functional materials. This review sheds light on recent progress in the postsynthetic modification of NGs and provides a brief summary of their production methods.
Ryo Sekiya, and Takeharu Haino, Chem. Asian J.,2020, 15, 2316-2328
●Supramolecular Polymerization and Functions of Isoxazole Ring Monomers

Head-to-tail dipole-dipole arrays of isoxazole rings lead to supramolecular helical assemblies where the assembly and disassembly are regulable based on temperature and solvent properties. The cooperative supramolecular polymerization characterized by a two-step polymerization consisting of nucleation and elongation is driven by the multiple dipole array as well as the induced dipoles in the supramolecular organization. The helical supramolecular assemblies are fabricated with the aid of multiple dipole–dipole interactions. The chiroptical properties, such as CD and CPL, are determined by the right-handed and left-handed helicities of the supramolecular organizations, which are directed by the stereogenic side chains. The AIE and AIEE are established in the supramolecular assemblies. The AIE feature of the platinum complex is inherent in the supramolecular assemblies, which results in luminogenic micelles. Emissive supramolecular micelles are fabricated. In this review, these aspects are briefly described, emphasizing the importance of the intermolecular dipole-dipole interactions in supramolecular chemistry.
Takeharu Haino and Takehiro Hirao, Chem. Lett., 2020, 49, 574-584.
●Upper-Rim Functionalization and Supramolecular Polymerization of a Feet-to-Feet-Connected Biscavitand
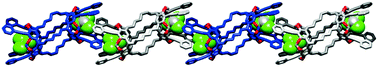
An octaiodobiscavitand was synthesized by an aromatic Finkelstein iodination reaction in good yield. Sonogashira and Suzuki coupling reactions of the octaiodobiscavitand gave rise to upper-rim-functionalized biscavitands that self-assembled to form the supramolecular polymer in the solid state.
Daisuke Shimoyama, Ryo Sekiya, and Takeharu Haino, Chem. Commun., 2020, 56, 3733-3736 .
●Folding control of a non-natural glycopeptide using saccharide-coded structural information for polypeptides
We synthesized “glyco-arylopeptides”, whose folding structure significantly changes depending on the kind of saccharide in their side chain. The saccharide moiety interacts with the main chain via hydrogen bonding, and the non-natural polypeptides form two well-defined architectures—(P)-31- and (M)-41-helices—depending on the length of the saccharide chains and even the configuration of a single stereo-genic center in the epimers.
Yuki Ishido, Naoya Kanbayashi, Naoka Fujii, Taka-aki Okayamura, Takeharu Haino, and Kiyotaka Onitsuka, Chem. Commun., 2020, 56, 2767-2770.
●A Regulable Internal Cavity Inside A Resorcinarene‐Based Hemi‐Carcerand
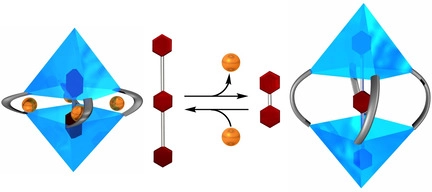
Covalent organic capsules, such as carcerands and hemi‐carcerands, constitute an interesting class of molecular hosts. These container molecules have confined spaces capable of hosting small molecules, although the size of the inner cavities cannot be changed substantially, limiting the scope of the applications. The title covalently linked container is produced by metal‐directed dimerization of a resorcinarene‐based cavitand possessing four 2,2’‐bipyridyl arms on the wide rim followed by olefin metathesis at the vertexes of the resulting capsule with a second‐generation Grubbs catalyst. The covalently linked bipyridyl arms permit expansion of the inner cavity by demetalation. This structural change influences the molecular recognition properties; the metal‐coordinated capsule recognizes only 4,4’‐diacetoxybiphenyl, while a metal‐free counterpart can encapsulate not only 4,4’‐diacetoxybiphenyl but also 2,5‐disubstituted‐1,4‐bis(4‐acetoxyphenylethynyl)benzene, which is 9.4 Å longer than the former guest. Molecular mechanics calculations predict that the capsule expands the internal cavity to encapsulate the long guest by unfolding the folded conformation of the alkyl chains, demonstrating the flexible and regulable nature of the cavity. The guest competition experiments show that the preferred guest can be switched by metalation and demetalation.
Kentaro Harada,. Ryo Sekiya, and Takeharu Haino, Chem. Eur. J., 2020, 26, 5810-5817(Cover Picture)
●トップダウン法により得られる化学修飾ナノグラフェン
Nanographenes, which are nanoscale graphene fragments, can be obtained by cutting off carbonaceous materials. This procedure, so-called top-down method, allows the gram-scale production of nanographenes. Our group has utilized these nanographenes for developing carbon-based functional materials. The installation of organic groups into the edge of nanographenes realized functionalized nanographenes. These nanographenes exhibit interesting properties, such as white-light emission, supramolecular polymerization and near-infrared emission.
Ryo Sekiya and Takeharu Haino, ファインケミカル, 2020年, 1月
●AIE-active Micells Formed by Self-assembly of an Amphiphilic Platinum Complex Possessing Isoxazole Moieties

An increased interest in the use of platinum complex based luminescent micelles for imaging biological tissues has emerged in recent years due to their low cytotoxicity, synthetic flexibility, photostability, and high emission efficiency. Here, we report a luminescent micelle that is prepared through the self-assembly of an amphiphilic, neutral Pt(II) complex with isoxazole moieties in THF/water on account of its aggregation-induced emission (AIE) property.
Takehiro Hirao, Hidemi Tsukamoto, Toshiaki Ikeda, and Takeharu Haino, Chem. Commun., 2020, 56, 1137-1140.
●Entropy–driven Cooperativity in the Guest Binding of an Octaphosphonate Biscavitand
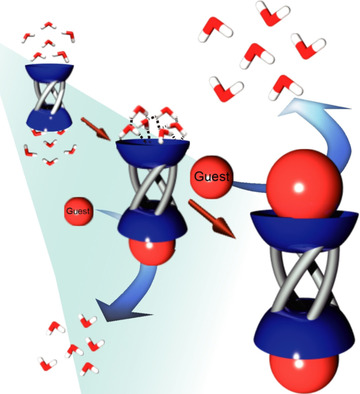
Uncommon entropy‐driven cooperativity is reported in the guest binding of an octaphosphonate biscavitand. Isothermal titration calorimetry analysis determined the thermodynamic parameters for the 1:2 host‐guest binding of biscavitands with ammonium guests in methanol, ethanol, isopropanol, and chloroform. Chloroform drove uncommon entropy‐driven cooperative binding, whereas the alcohols resulted in enthalpy‐driven noncooperative binding. 1H NMR studies revealed that each cavity possessed six water molecules in chloroform, which were liberated upon the guest binding. The enthalpy‐entropy compensation relationship produced a large positive intrinsic entropy in chloroform, which implies that water desolvation causes a considerable entropic gain by paying an enthalpic penalty due to breaking the hydrogen bonding networks of the water clusters.
Daisuke Shimoyama and Takeharu Haino, Chem. Eur. J., 2020, 26, 3074-3079(Cover Picture)
●Chirality-Embedded Nanographene
The development of chiral nanographenes hasmostly been carried out by bottom-up methods and examplesof species developed by the post-modification of nanogra-phenes prepared by top-down methods remain limited. Weshow that the attachment of chiral functional groups onto theedge of nanographenes generates chirality on the surface. X-ray diffraction analysis and DFT calculations indicate that thechirality of the functional groups is transferred to the surfacevia steric interactions from the chiral center through the five-membered cyclic imide to the nanographene edge. The excitoncoupling between the p-bromophenyl groups confirms that thefunctional groups are arranged on the armchair edges atdistances that permit exciton coupling, which provides infor-mation about their relative orientation. These pieces ofinformation help to elucidate the edge structure of nano-graphenes prepared by top-down methods.
Shohei Nishitani, Ryo Sekiya, and Takeharu Haino, Angew. Chem. Int. Ed., 2020, 59, 669-673(Hot Paper)
●Feet-to-feet Connected Trisresorcinarenes
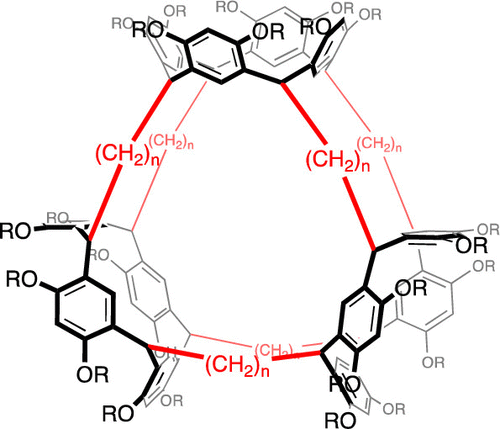
The macrocyclization of resorcinol and odd-numbered bisdioxolanes under acidic conditions produced feet-to-feet connected trisresorcinarenes possessing three resorcinarene units linked with odd-numbered alkyl chains. The formation of trisresorcinarenes was confirmed using high-resolution mass spectrometry and NMR spectroscopy. The trisresorcinarenes were isolated as protected forms in moderate yields. The protected trisresorcinarenes exhibited D3h symmetry in conformation in solution. Crystal structure analysis revealed that the protected trisresorcinarenes possess large inner spaces surrounded by three resorcinarene units.
Daisuke Shimoyama, Ryo Sekiya, Hiroto Kudo, and Takeharu Haino, Org. Lett., 2020, 22, 352-356(Cover Picture)
DOI:10.1021/acs.orglett.9b0369
2019
●A Protocol for Separation of Nanographenes
Top-down methods are convenient preparative methods for nanographenes, although the products consist of graphene fragments with a broad size distribution. We show that a combination of dialysis membranes (50, 25, 15, 8, and 2 kD) can conveniently separate nanographenes into five size distributions. The separated nanographenes can be employed as starting materials for carbon-based functional materials.
Matsumoto, Ikuya; Sekiya, Ryo;Haino, Takeharu, RSC Adv ., 2019, 9, 33843-33846. (Hot Article)
●Conformational Characteristics of Feet-to-Feet-Connected Biscavitands
X-ray crystallography of an acetoxy-protected bisresorcinarene and biscavitands possessing phosphonate and dialkylsilyl bridges revealed that the bisresorcinarene and the biscavitands adopt helical forms in the solid state. Helical conformations were also found in solution. The helix–helix interconversions of the biscavitands occurred with high activation barriers of more than 50 kJ mol–1. The activation parameters of the helix–helix interconversions were determined using exchange spectroscopy (EXSY). The positive activation enthalpies and the negative activation entropies suggest that the transition states of the helix–helix interconversion process are most likely more strained and symmetric than the ground states. The compensatory enthalpy–entropy correlation is found in the series of activation parameters, giving rise to a compensation temperature of 254 K.
Shimoyama, Daisuke; Haino,Takeharu, J. Org. Chem ., 2019 , 84 , 13483-13489(Cover Picture)
●Intrinsic Emission from Nanographenes
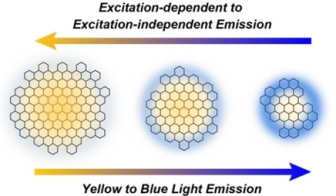
Top‐down approaches have been widely used as convenient methods for the production of nanographenes. To understand the photoemission properties of nanographenes, their separation and the optical properties of the individual fractions is important. By using a combination of size‐exclusion and silica‐gel‐adsorption chromatography, we separated lipophilic nanographenes that contained para‐methoxybenzyl groups. The mixture consisted of large (average 19.8 nm) and small (average 4.9 nm) nanographenes, whilst unreacted carboxy groups remained in the latter group. Optical measurements revealed that oxygen‐containing functional groups had little influence on the photoemission of the nanographenes, thus indicating that the intrinsic emission, that is, emission from the sp2 surfaces, was responsible for the photoemission. Two photoemission bands were observed for all of the fractions, which likely originated from the edge and inner parts of nanographene.
Yamato, Kairi; Sekiya, Ryo; Nishitani, Shohei; Haino, Takeharu, Chem. Asian. J. , 2019 , 14 , 3213-3220.
●Helical Assembly of a Dithienogermole Exhibiting Switchable Circularly Polarized Luminescence

Dithienogermole derivatives S- and R-1 possessing phenylisoxazoles and chiral side chains were synthesized. The helical assembly of 1 in methylcyclohexane exhibited circularly polarized luminescence (CPL). The CPL signals of the assembly in the elongation regime were inverted with respect to those in the nucleation regime.
Hirano, Kyohei;Ikeda, Toshiaki; Fujii, Naoka; Hirao, Takehiro; Nakamura, Masashi; Adachi, Yohei; Ohshita, Joji; Haino, Takeharu, Chem. Commun ., 2019 , 55 ,10607-10610
●Ring-Chain Competition in Supramolecular Polymerization Directed by Molecular Recognition of Bisporphyrin Cleft

Increasing interest in innovative supramolecular materials has spurred efforts to develop head-to-tail monomers possessing a host moiety as a head and a guest moiety as a tail, making them capable of forming supramolecular polymers through intermolecular associations while avoiding intramolecular cyclization. This competition between the intramolecular cyclization and the intermolecular association is influenced by conformational entropy, relying on the flexibility of the linker chain that connects the host moiety to the guest moiety in a head-to-tail monomer. However, there are limited reports presenting a quantitative thermodynamic picture describing the conformational entropy in ring–chain equilibrium processes. Here, we report the quantitative evaluation of the role of conformational entropy in the ring–chain equilibrium mechanism of the supramolecular polymerization of a head-to-tail monomer possessing a bisporphyrin host and a trinitrofluorenone guest connected with various alkyl chains as linkers. The supramolecular polymerization of the head-to-tail monomers was studied using UV/vis, NMR, and diffusion-ordered spectroscopy spectroscopic techniques and viscometry. The thermodynamic parameters of the ring–chain equilibrium were determined in the initial stage of the supramolecular polymerization. The conformational entropy, which relies on the flexibility of the linker, had a significant influence on the critical polymerization concentration. This quantitative discussion of ring–chain competition is expected to provide a foundation for the proper design of artificial head-to-tail monomers that form supramolecular polymers.
Hirao, Takehiro; Hisano Naoyuki; Akine Shigehisa; Kihara Shin-ichi; Haino, Takeharu, Macromolecules, 2019, 52, 6160-6168.
DOI: 10.1021/acs.macromol.9b01012
●Near-Infrared Emitting Nitrogen-Doped Nanographenes
The quantum‐size effect, which enables nanographenes to emit photoluminescence (PL) in the UV to visible region, has inspired intense research. However, the control of the PL properties of nanographenes through manipulation of their π‐system by post‐modifications is not well developed. By utilizing a ring‐closure reaction between an aromatic 1,2‐dicarboxylic acid and a 1,8‐naphthalenediamine derivative, which produces a perimidine framework, nitrogen‐doped nanographenes were realized. Two nanographenes produced by a one‐pot reaction of edge‐oxidized nanographene (GQD‐2) with 1,8‐naphthalenediamine derivatives (GQD‐1a and GQD‐1b) displayed an absorption band extending to >1000 nm; furthermore, the PL wavelength of GQD‐1a was significantly red‐shifted into the near‐infrared (NIR) region in which it can be used for bioimaging. Time‐dependent DFT calculations of model nanographenes showed that the functional groups narrow the HOMO–LUMO gap, realizing the NIR‐emitting nanographenes.
Yamato, Kairi; Sekiya, Ryo; Suzuki, Kaho; Haino, Takeharu, Angew. Chem. Int. Ed. 2019, 58, 9022-9026
●Substituent-Controlled Racemization of Dissymmetric Coordination Capsules
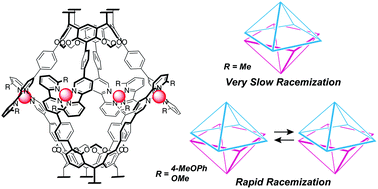
We report the effect of substituents (methyl, isopropyl, methoxy, and methoxyphenyl) at the 6′-position of the 2,2′-bipyridyl arms on the racemization of dissymmetric coordination capsules 1a–d. When the capsules included (R)-4,4′-diacetoxy-2,2′-benzyloxycarboxyl-biphenyl ((R)-3), the (M)-helical conformer was enriched with a diastereomeric excess (de%) of >98% for 1a, 31% for 1b, 81% for 1c and 75% for 1d. The entrapped guests in 1a, 1c and 1d can be removed by washing the solid containing the host–guest complexes with diethyl ether. The rate of racemization in THF follows the order of 1c > 1d ≫ 1a. X-ray crystal structural analysis and density functional theory calculation of model complex 4c indicate a distorted tetrahedral coordination of the Cu(I) center, and UV-vis absorption spectroscopy indicates similar coordination environments in 1c and 4c. A series of experiments demonstrates that the racemization rate depends on the dihedral angles of the bipyridyl arms, and the angles are regulated by the substituents. The methoxy and methoxyphenyl substituents in 1c and 1d enlarge the dihedral angles of the bipyridyl arms. This facilitates the access of solvent molecules to the Cu(I) centers and promotes racemization. The slower racemization of 1d can be ascribed to the steric protection of the Cu(I) centers from incoming solvent molecules by the p-methoxyphenyl group.
Harada, Kentaro; Sekiya, Ryo; Maehara, Takeshi; Haino, Takeharu, Org. Biomol. Chem., 2019, 17, 4729-4735(Cover Picture)
●Tunable Enforced Cavities inside Self-assembled Capsules
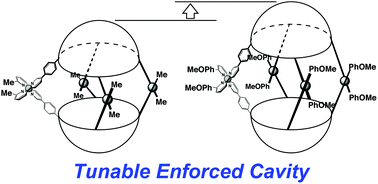
Controlling and tuning the molecular recognition properties is a crucial task in host–guest chemistry. The size and dimension of the guest-binding space inside self-assembled capsules 1a–c is successfully determined by installing the substituents at the 6′-position of 2,2′-bipyridyl arms. X-ray diffraction analysis and DFT calculations at the M06-2X/6-31G(d,p)+LanL2DZ level of theory demonstrate that the p-methoxyphenyl group expands the dihedral angle of the 2,2′-bipyridyl arms through its π-stacking interaction to the 2,2′-bipyridyl arm, whereas the steric interaction of the isopropyl group to the neighboring bipyridyl arm slightly reduces the dihedral angle of the two bipyridyl groups. The Cu(I)-united 2,2′-bipyridyl arms function as a hinge; accordingly, installing the p-methoxyphenyl group extends the cavity by ca. 2 Å, whereas the isopropyl group shrinks the cavity more than that of the capsule 1a with methyl groups at the 6′-positions. These steric interactions influence the molecular recognition of the capsules 1a–c for rigid ditopic guest 2a as well as flexible ones 2b–f. The guest selectivity inside the enforced cavity is determined by varying the substituents.
Maehara, Takeshi; Sekiya, Ryo; Kentaro, Harada; Haino, Takeharu, Org. Chem. Front. 2019, 6, 1561-1566(Cover Picture)
●Separation of Spectroscopically Uniform Nanographenes
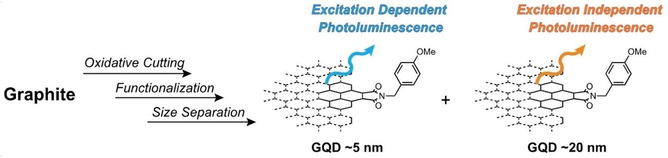
Excitation‐dependent photoluminescence (PL) is a well‐known property of graphene quantum dots (GQDs). For the development of carbon‐based photofunctional materials, GQDs possessing uniform PL properties are in high demand. A protocol has been established to separate spectroscopically uniform lipophilic GQD‐1a from a mixture of GQD‐1 mainly composed of GQD‐1a and GQD‐1b. The mixture of GQD‐1 was synthesized through the reaction of p‐methoxybenzylamine with GQD‐2 prepared from graphite by common oxidative exfoliation. Size‐exclusion chromatography gave rise to GQD‐1a and GQD‐1b, with diameters of 19.8 and 4.9 nm, respectively. Large GQD‐1a showed that the PL was fairly independent of the excitation wavelengths, whereas the PL of small GQD‐1b was dependent on excitation. The excitation‐dependent nature is most likely to be associated with the structures of sp2 domains on the graphene surfaces. The large sp2‐conjugated surface of GQD‐1a is likely to possess well‐developed and large sp2 domains, the band gaps of which do not significantly vary. The small sp2‐conjugated surface of GQD‐1b produces small sp2‐conjugated domains that generate band gaps differing with domain sizes.
Yamato, Kairi; Sekiya, Ryo; Abe, Manabu; Haino, Takeharu, Chem. Asian. J., 2019, 14, 1786-1791(Cover Picture)
●Organogelators of 5,17-Difunctionalized Calix[4]arenes

5,17-Difunctionalized calix[4]arenes 1a,b were found to function as low molecular-weight organic gelators. They formed organogels with a variety of organic solvents. SEM images showed 1a formed stacked structures in the xerogels. Single-crystal X-ray analysis of 1c as well as AFM, XRD, and SAXS measurements of 1a indicated that the xerogels contained lamellar structures of the calix[4]arenes.
Lai, Nang, Duy; Sekiya, Ryo; Tosaka, Masatoshi; Yamago, Shigeru; Matsumoto, Takuya; Nishino, Takashi; Ichikawa, Takayuki; Haino, Takeharu, Chem. Lett., 2019, 48, 43-46.
●Designer Supramolecular Polymers with Specific Molecular Recognitions

Supramolecular polymers are members of an emergent class of polymer materials that exhibit designability and flexibility. This article describes how supramolecular polymers can be synthesized by taking advantage of our host–guest structures based on a calix[5]arene, a bisporphyrin, and a self-assembled capsule. Linear and two-dimensional fullerene nanostructures can be fabricated using the designed monomer structures. The porphyrin donor–acceptor interaction directs the supramolecular polymerization, resulting in linear porphyrin polymers that behave similarly to a conventional polymer chain in solution, even though their structures are dynamic and time-averaged. The fragile supramolecular polymer chains are cross-linked to fabricate a robust self-standing film. The sequence reorganization of the supramolecular homopolymer is established by competitive ditopic guest complexation. The sequence-controlled terpolymer is fabricated via self-sorting behavior. The postmodifications of the supramolecular polymer chains, as well as the polymer chains themselves, are achieved by grafting and non-covalent cross-linking to regulate the macroscopic properties and structures of the polymer main chains. These uniquely organized polymers are fabricated on a nanoscale.
Haino, Takeharu, Polym. J., 2019, 51, 303-318
2018
●Facile Synthesis of an Eight-Armed Star-Shaped Polymer via Coordination-Driven Self-Assembly of a Four-Armed Cavitand
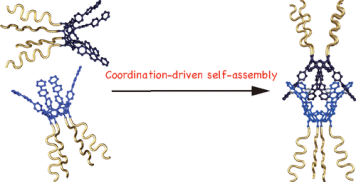
The polystyrene chains were installed at the lower rim of a resorcinarene-based cavitand via reversible addition–fragmentation (RAFT) polymerization to form a four-armed star-shaped polymer. A star-shaped polystyrene-functionalized supramolecular capsule was prepared through the coordination-driven self-assembly of the four-armed start-shaped polymer with silver ions. The eight-armed start-shaped supramolecular capsule encapsulated 4,4′-diacetoxybiphenyl as did a cavitand-based self-assembled capsule. A DOSY measurement indicated that the eight-armed star-shaped polymer was twice as large as the four-armed star-shaped polymer. The solution behaviors of these compounds resulted in a difference in their zero-shear viscosities.
Nitta, Natsumi; Mei, Takatsuka; Kihara, Shin-ichi; Sekiya, Ryo; Haino, Takeharu, ACS Macro. Lett., 2018, 7, 1308-1311(Cover Picture)
DOI:10.1021/acsmacrolett.8b00669
●Majority-Rules Effect and Allostery in Molecular Recognition of Calix[4]arene-Based Triple-Stranded Metallohelicates
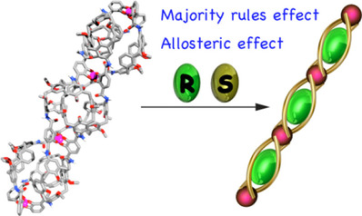
The triple‐stranded metallohelicates 1a,/1b–3a/3b possessing internal guest binding cavities surrounded by calix[4]arene units were synthesized through coordination‐driven self‐assembly. UV/Vis titration experiments verified that the metallohelicates encapsulated N‐methyl pyridinium cations bearing amino acid groups to form host–guest complexes. The guest chirality was transferred to the helicity of the helicates through the steric contact between the stereogenic center of the amino acid group and the metal cores. The (M) helicity was induced when guests the (R)‐4‐‐(R)‐6 were accommodated within the cavities. The multiple guest complexation within the self‐assembled helicates 2a and 3a displayed large positive cooperative effects, indicating that the first guest complexation preorganizes the rest of the cavities to facilitate a subsequent guest binding. This cooperativity results in the majority‐rules effect in the chiral guest binding for 2a and 3a.
Yamasaki, Yutaro; Shio, Hidemi; Amimoto, Tomoko; Sekiya, Ryo; Haino, Takeharu, Chem. Eur. J., 2018, 24, 8558-8568
●Supramolecular Properties of a Monocarboxylic Acid-Functionalized “Texas-Sized” Molecular Box
A new carboxylic acid-functionalized “Texas-sized” molecular box TxSB-CO2H has been prepared by combining two separate building blocks via an iodide-catalyzed macrocyclization reaction. A single-crystal X-ray diffraction analysis revealed a paired “clip-like” dimer in the solid state. Concentration-dependent behavior is seen for samples of TxSB-CO2H as prepared, as inferred from 1H NMR spectroscopic studies carried out in DMSO-d6. However, in the presence of excess acid (1% by weight of deuterated trifluoracetic acid; TFA-d1), little evidence of aggregation is seen in DMSO-d6 except at the highest accessible concentrations. In contrast, the conjugate base form, TxSB-CO2–, produced in situ via the addition of excess triethylamine to DMSO-d6 solutions of TxSB-CO2H acts as a self-complementary monomer that undergoes self-assembly to stabilize a formal oligomer ([TxSB-CO2–]n) with a degree of polymerization of approximately 5–6 at a concentration of 70 mM. Evidence in support of the proposed oligomerization of TxSB-CO2– in solution and in the solid state came from one- and two-dimensional 1H NMR spectroscopy, X-ray crystallography, dynamic light scattering (DLS), and scanning electron microscopy (SEM). A series of solution-based analyses carried out in DMSO and DMSO-d6 provide support for the notion that the self-assembled constructs produced from TxSB-CO2– are responsive to environmental stimuli, including exposure to the acetate anion (as its tetrabutylammonium, TBA+, salt), and changes in overall concentration, temperature, and protonation state. The resulting transformations are thought to reflect the reversible nature of the underlying noncovalent interactions. They also permit the stepwise interconversion between TxSB-CO2H and [TxSB-CO2–]n via the sequential addition of triethylamine and TFA-d1. The present work thus serves to illustrate how appropriately functionalized molecular box-type macrocycles may be used to develop versatile stimuli-responsive materials. It also highlights how aggregated forms seen in the solid state are not necessarily retained under competitive solution-phase conditions.
Wu, Ren-Tsung;Chi, Xiaodongi; Hirao, Takehiro;Lynch, Vincent M. Lynch; Sessler, Jonathan L, J. Am. Chem. Soc., 2018, 140, 6823-6831.
●Control over Multiple Molecular States with Directional Changes Driven by Molecular Recognition
Recently, ligand–metal coordination, stimuli-responsive covalent bonds, and mechanically interlinked molecular constructs have been used to create systems with a large number of accessible structural states. However, accessing a multiplicity of states in sequence from more than one direction and doing so without the need for external energetic inputs remain as unmet challenges, as does the use of relatively weak noncovalent interactions to stabilize the underlying forms. Here we report a system based on a bispyridine-substituted calix[4]pyrrole that allows access to six different discrete states with directional control via the combined use of metal-based self-assembly and molecular recognition. Switching can be induced by the selective addition or removal of appropriately chosen ionic guests. No light or redox changes are required. The tunable nature of the system has been established through a combination of spectroscopic techniques and single crystal X-ray diffraction analyses. The findings illustrate a new approach to creating information-rich functional materials.
Hirao, Takehiro; Kim, Dong Sub; Chi, Xiaodong;Lynch Vincent M.;Ohara, Kazuaki; Park, Jung Su; Yamaguchi, Kentaro; Sessler, Jonathan L., Nature Commun.,2018,9, 823
DOI:10.1038/s41467-018-03220-0
●Supramolecular Copolymerization by Sequence Reorganization of a Supramolecular Homopolymer

The homopolymeric sequence formed by the head‐to‐head association of tetrakisporphyrin 1 is completely dissociated by the competitive association of the ditopic guest G2, resulting in the supramolecular copolymer poly‐1⋅G2 with an alternatingly repeating host–guest sequence. The 1:1 stoichiometry of 1 and G2 is confirmed by a Job plot using UV/Vis titration and diffusion‐ordered NMR spectroscopy (DOSY). The solution viscometry for poly‐1 and poly‐1⋅G2 suggests that the supramolecular chain of poly‐1 behaves like a rod, whereas the supramolecular copolymer chain of poly‐1⋅G2 behaves like a swelled fat chain, which is entangled in the semi‐dilute regime. Atomic force microscopy shows that the supramolecular polymer poly‐1⋅G2 is highly oriented through the interdigitation of the long alkyl chains.
Nadamoto, Kouhei; Maruyama, Kei; Fujii, Naoka; Ikeda, Toshiaki; Kihara, shin-ichi; Haino, Takeharu, Angew. Chem. Int. Ed.,2018,57, 7028-7033
●Controllable Direction of Porphyrin Derivatives in Two Cyclodextrin Cavities
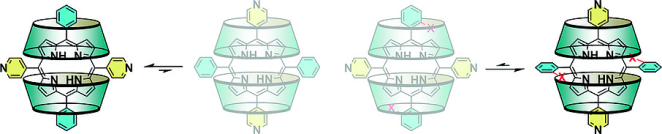
Porphyrin–trimethyl‐β‐cyclodextrin (TMe‐β‐CDx) complexes have pseudorotaxane structures in which two meso‐phenyl and/or ‐pyridyl moieties penetrate the upper rim of two TMe‐β‐CDx molecules. Porphyrin derivatives with one to three pyridyl moieties at meso positions formed complexes with TMe‐β‐CDx in which the penetration of the upper rim of the two TMe‐β‐CDxs by the pyridyl moieties was minimized. In contrast, in TMe‐β‐CDx complexes formed with porphyrin derivatives bearing two 2‐methoxyphenyl moieties and two pyridyl moieties, the pyridyl moieties penetrate the upper rim of the two molecules because steric hindrance prevents penetration by the 2‐methoxyphenyl moieties.
Horiguchi, Banri; Nakaya, Toshimi; Ueda, Masafumi; Sugisawa, Kouta; Mizuta, Tsutomu; Haino, Takeharu; Kawata, Naomi; Ikeda, Atsushi, Eur. J. Org. Chem.,2018,18, 2138-2143
●Pseudorotaxanes in the Gas Phase: Structure and Energetics of Protonated Dibenzylamine-crown Ether Complexes
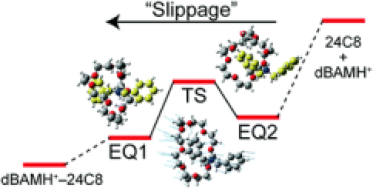
We observe UV spectra of protonated dibenzylamine (dBAMH+) and its complexes with 15-crown-5 (dBAMH+–15C5), 18-crown-6 (dBAMH+–18C6), and 24-crown-8 (dBAMH+–24C8) under cold (∼10 K) gas-phase conditions by UV photodissociation (UVPD) and UV–UV hole-burning (HB) spectroscopy. The UVPD spectrum of the dBAMH+–15C5 complex shows an extensive low-frequency progression, which originates from a unique conformation of the dBAMH+ part with benzene rings facing closely to each other, while UVPD and calculation results suggest open conformations of the dBAMH+ part for dBAMH+–18C6 and dBAMH+–24C8. UV–UV HB spectra of the dBAMH+–24C8 complex indicate that there exist at least two conformers; multiple conformations can contribute to high stability of dBAMH+–24C8 pseudorotaxane due to “conformational” entropic effects. The UVPD experiment indicates that the dissociation probability of dBAMH+–24C8 into dBAMH+ and 24C8 is substantially smaller than that of dBAMH+–15C5 and dBAMH+–18C6, which can be related to the barrier height in the dissociation process. The energetics of the dBAMH+–24C8 complex is investigated experimentally with NMR spectroscopy and theoretically with the global reaction route mapping (GRRM) method. An energy barrier of ∼60 kJ mol−1 is present in the pseudorotaxane formation in solution, whereas there is no barrier in the gas phase. In the course of the photodissociation, excited dBAMH+–24C8 complexes can be trapped at many local minima corresponding to multiple conformations. This can result in effective dissipation of internal energy into degrees of freedom not correlated to the dissociation and decrease the dissociation probability for the dBAMH+–24C8 complex in the gas phase. The energy barrier for the pseudorotaxane formation in solution originates not simply from the slippage process but rather from solvent effects on the dBAMH+–24C8 complex.
Kida, Motoki; Shimoyama, Daisuke; Ikeda, Toshiki; Sekiya, Ryo; Haino, Takeharu; Ebata, Takayuki; Jouvet, Christophe; Inokuchi, Yoshiya, Phys. Chem. Chem. Phys.,2018,20, 18678-18687.
'
●A Supramolecular Polymer Network of Graphene Quantum Dots
Graphene quantum dot (GQD)–organic hybrid compounds (GQD‐2b–e) were prepared by introducing 3,4,5‐tri(hexadecyloxy)benzyl groups (C16) and linear chains terminated with a 2‐ureido‐4‐[1H]‐pyrimidinone (UPy) moiety onto the periphery of GQD‐1. GQD‐2b–e formed supramolecular assemblies through hydrogen bonding between the UPy units. GPC analysis showed that GQDs with high loadings of the UPy group formed larger assemblies, and this trend was confirmed by DOSY and viscosity measurements. AFM images showed the polymeric network structures of GQD‐2e on mica with flat structures (ca. 1.1 nm in height), but no such structures were observed in GQD‐2a, which only carries the C16 group. GQD‐2c and GQD‐2d formed organogels in n‐decanol, and the gelation properties can be altered by replacing the alkyl chains in the UPy group with ethylene glycol chains (GQD‐3). GQD can thus be used as a platform for supramolecular polymers and organogelators by suitable chemical functionalization.
Uemura, Yuichiro; Yamato Kairi; Sekiya, Ryo; Haino, Takeharu, Angew. Chem. Int. Ed.,2018,57, 4960-4964
●Synthesis and Dimerization Studies of a Lipophilic Photoresponsive Aryl Extended Tetraureacalix[4]pyrrole
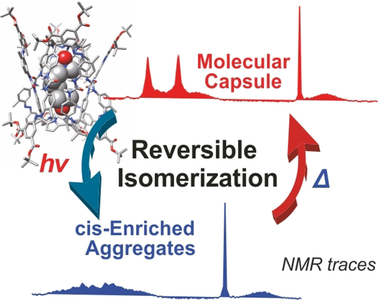
We describe the syntheses of the lipophilic aryl‐extended α,α,α,α‐tetraurea‐phenyl‐calix[4]pyrrole 1, featuring four appended azo‐phenyl groups with two tert‐butoxy carbonyl meta‐substituents and its photo‐inactive counterpart 2. In CD2Cl2 solutions, both tetraurea‐calix[4]pyrroles self‐assemble into dimeric capsules by encapsulating one molecule of a suitable bis‐N‐oxide or two molecules of a mono‐N‐oxide. The dimeric capsules are mainly stabilized by a cyclic array of sixteen hydrogen bonds established between the eight unidirectionally oriented urea groups. Photoirradiation experiments demonstrated the trans‐to‐cis isomerization of the azo‐phenyl groups and the formation of a plethora of stereo isomeric cis‐azo‐enriched capsular assemblies. The highly cis‐azo enriched capsular assemblies seem to show a reduced stability and their involvement in equilibria with non‐capsular counterparts that also bind the N‐oxides. The thermally induced cis‐to‐trans interconversion processes demonstrated the reversibility of the photoisomerization and the photostability of most binding partners. An equimolar mixture of the two tetraureas produced two homodimeric capsules and the heterodimeric counterpart in a ratio close to statistical distribution.
Sekiya, Ryo; Díaz-Moscoso, Alejandro; Ballester, Pablo, Chem. -Eur. J., 2018, 24, 2182-2191
●A Circularly Polarized Luminescent Organogel Based on a Pt(II) Complex Possessing Phenylisoxazoles
A Pt(II)phenylbipyridine complex 1, possessing phenylisoxazoles, long alkyl chains, and chiral alkyl chains, was synthesized. Complex 1 formed a stacked assembly in chloroform, self-assembled in a cooperative manner in methylcyclohexane (MCH), and gelled in higher alcohols and dodecane. In the nucleation regime, 1 assembled via a Pt–Pt interaction, which was demonstrated by the metal–metal-to-ligand charge transfer (MMLCT) emission band of the assembly. In the elongation regime, 1 displayed aggregation-induced emission enhancement (AIEE) character. The assembly of 1 formed in MCH was chiroptically non-active, suggesting that the assembly was non-helical. Complex 1 also exhibited strong AIEE and circularly polarized luminescence (CPL) with an anisotropic factor (glum) of 0.011 in 1-decanol gel, indicating that a chiral assembly was formed in the gel.
Ikeda, Toshiaki; Hirano, Kyohei; Haino, Takeharu, Mater. Chem. Front., 2018, 2, 468-474(Cover Picture)
2017
●Synthesis and Sturcture of Feet-to-Feet Connected Bisresorcinarenes

Bisresorcinarenes 1a–d were obtained in excellent yields, and 1e was finally
obtained in 50% yield. X-ray diffraction analysis showed that 1a and 1b
adopted helical conformations, whereas the two resorcinarenes of 1c–e were
in parallel orientations in which the clefts of the aliphatic chains entrapped
one or two solvent molecules. The conformational study revealed that the
helix interconversion between the (P)- and (M)-helical conformers depended
on the length of the aliphatic chains. 1a had the largest energetic barrier
to helix interconversion, while in 1b, its more flexible aliphatic chains
lowered its energetic barriers. The P/M interconversion of 1a was coupled
with the clockwise/anticlockwise interconversion of the interannular hydrogen
bonding of the two resorcinarenes. The large negative entropic contributions
indicate that the transition state is most likely more ordered than the
ground states, suggesting that the transition state is most likely symmetric
and is solvated by water molecules. Calculations at the M06-2X/6-31G(d,p)
level revealed that the more stable (P)-conformation has clockwise interannular
hydrogen bonding between the two resorcinarenes.
Shimoyama, Daisuke; Ikeda, Toshiaki; Sekiya, Ryo; Haino, Takeharu, J. Org. Chem., 2017, 82, 13220-13230
●Hexameric Assembly of 5,17-di-Subsituted Calix[4]arene intheSolid State
Chiral 5,17-difunctionalized-25,26,27,28-tetrapropyloxycalix[4]arene possessing (S)-mandelamide arms ((S,S)-1) afforded cocrystals (S,S)-1·(solvent) (solvent = MeOH, EtOH, 1-PrOH, 2-PrOH, and CH3CN). X-ray diffraction analysis revealed that (S,S)-1 formed head-to-tail columnar structures. In most cocrystals (MeOH, EtOH, 1-PrOH, and CH3CN), the columnar structures were arranged hexamerically to form a chiral hexagonal channel, which is a rare example of calix[4]arene derivatives in the solid state. The fact that the branched guest (2-PrOH) and the racemic mixture of the host formed different crystal packings wherein no hexameric structure existed indicates that both the linearity of the guest and the homochirality of the host are important factors for driving the columnar structures to form the hexameric assembly. Apohost (S,S)-1apo showed interesting adsorption behavior; it adsorbed benzene among the organic molecules tested. The adsorption and desorption processes were repeated several times, demonstrating the sponge-like character of (S,S)-1apo.
Yamasaki, Yutaro; Sekiya, Ryo; Haino, Takeharu, CrystEngComm, 2017, 19, 6744-6751(Cover Picture)
●Slow IntermolecularComplexation-Decomplexation Exchanges of Cyclodextrins in Fullerene and Its DerivativeComplexes
Fullerenes (C60 and C70) and several functionalized C60 derivatives can be encapsulated in two γ‐cyclodextrins (γ‐CDxs). Although intermolecular complexation–decomplexation exchange of γ‐CDx is known to be very slow, the exchange rates have yet to be quantitatively measured. Herein, we determined that the pseudo‐first‐order association and dissociation rate constants for the γ‐CDx2•C70 complex were 4.3 and 0.6 s−1 at 23 °C, respectively. In contrast, the intermolecular exchange rates for the γ‐CDx2•C60 and C60 derivative complexes were slower than the time scale of exchange spectroscopy NMR experiments and the exchange rate constants were in the order of 10−4 s−1. Furthermore, a γ‐CDx2•C60 derivative complex was shown to have different intermolecular exchange rates for the two γ‐CDxs depending on steric hindrance from the substituent on the C60 derivative.
Ikeda, Atsushi; Mae, Tomoya; Sugikawa, Kouta; Komaguchi, Kenji; Konishi, Toshifumi; Hirao, Takehiro; Haino Takeharu, Chemistry Select, 2017, 2, 11322-11327.
●Sequence-Controlled Supramolecular Terpolymerization Directed by Specific Molecular Recognitions
Nature precisely manipulates primary monomer sequences in biopolymers.
In synthetic polymer sequences, this precision has been limited because
of the lack of polymerizationtechniques for conventional polymer synthesis.
Engineering the primary monomer sequence of a polymer main chain represents
a considerable challenge in polymer science. Here, we report the development
of sequence-controlled supramolecular terpolymerization via a self-sorting
behavior among three sets of monomers possessing mismatched host–guest
pairs. Complementary biscalix[5]arene-C60, bisporphyrin-trinitrofluorenone
(TNF), and Hamilton’s bis(acetamidopyridinyl)isophthalamide-barbiturate
hydrogen-bonding host–guest complexes are separately incorporated into
heteroditopic monomers that then generate an ABC sequence-controlled supramolecular
terpolymer. The polymeric nature of the supramolecular terpolymer is confirmed
in both solution and solid states. Our synthetic methodology may pave an
avenue for constructing polymers with tailored sequences that are associated
with advanced functions.
Hirao, Takehiro; Kudo, Hiroaki; Amimoto, Tomoko; Haino Takeharu, Nat. Commun.,2017,8, Article No. 634
DOI: 10.1038/s41467-017-00683-5
●Induced dipole-directed cooperative self-assembly of a benzotrithiophene
Abstract:A benzotrithiophene derivative possessing phenylisoxazoles self-assembled to form stacks. The molecule isodesmically self-assembled in chloroform, whereas it self-assembled in a cooperative fashion in decalin and in methylcyclohexane. Thermodynamic studies based on isodesmic, van der Schoot, and Goldstein–Stryer mathematical models revealed that the self-assembly processes are enthalpically driven and entropically opposed. An enthalpy–entropy compensation plot indicates that the assembly processes in chloroform, decalin, and methylcyclohexane are closely related. The enthalpic gains in less-polar solvents are greater than those in more-polar solvents, resulting in the formation of large assemblies in decalin and in methylcyclohexane. The formation of large assemblies leads to cooperative assemblies. The elongation process is enthalpically more favored than the nucleation process, which drives the cooperativity of the self-assembly. DFT calculations suggested that a hexameric assembly is more stable than tetrameric or dimeric assemblies. Cooperative self-assemblies based on intermolecular interactions other than hydrogen bonding have rarely been reported. It is demonstrated herein that van der Waals interactions, including induced dipole–dipole interactions, can drive the cooperative assembly of planar π-conjugated molecules.
Ikeda, Toshiaki;Adachi, Hiroaki; Fueno, Hiroyuki; Tanaka, Kazuyoshi; Haino, Takeharu, J. Org. Chem., 2017, 82, 10062-10069.
●Supramolecular Polymeric Assemblies of pi - Conjugated Molecules Possessing Phenylisooxazoles
Abstract:Supramolecular polymeric stacks of flat molecular components provide a highly ordered molecular organization that results in unique functions. The engineering of molecular organization, therefore, has attracted a great deal of attention both in supramolecular chemistry and in material science. The electronic and structural properties of molecular components determine the forthcoming supramolecular organization structure and properties. Non-covalent intermolecular interactions are responsible for directional growth in supramolecular polymerization. We have discovered that a phenylisoxazole ring system drives supramolecular polymerization in a directional way. π-Conjugated molecular components possessing phenylisoxazole moieties constructively assemble to form one-dimensional stacked assemblies via π–π stacking and dipole–dipole interaction. The introduction of a chirality onto the molecular components results in supramolecular helical organizations with unique photophysical functions. Switchable circularly polarized luminescence (CPL) and circular dichroism (CD) are developed in the supramolecular helical organizations. The grown assemblies provide highly entangled fibrillar networks, giving rise to organogels. The photo-responsive organogels and toroidal nanostructures are fabricated with the assistance of a photo-addressable azobenzene group. A light-harvesting system is developed in the organogels.
Ikeda, Toshiaki; Haino, Takeharu, Polymer,2017,128, 243-256
DOI: 10.1016/j.polymer.2017.02.05
●Photoluminescence Response of Graphene Quantom Dots toward Organic Bases and an Acid
Edge-modified graphene quantum dots (GQD-1) showed base-dependent photoluminescence (PL) responses. DBN, DBU, and Et3N gradually enhanced the PL intensity and then caused quenching, whereas quenching was not observed when pyridine and pyrimidine were used. The quenching can be explained by the photoinduced electron transfer from the anchored bases at the periphery to the emissive sites of GQD-1.
Suzuki, Kaho; Yamato, Kairi; Sekiya Ryo; Haino, Takeharu, Photochem. Photobio. Sci.2017,16, 623-626
●Supramolecular Graft Copolymerization of a Polyester via Guest-Selective Encapsulation of a Self-Assembled Capsule
Repeating guest units of polyesters poly‐(R)‐2 were selectively encapsulated by capsule 1(BF4)4 to produce supramolecular graft polymers. The encapsulation of the guest units was confirmed by 1H NMR spectroscopy. The graft polymer structures were confirmed by the increase in the hydrodynamic radii and the solution viscosities of the polyesters upon complexation of the capsule. After the capsule was formed, atomic force microscopy showed extension of the polyester chains. The introduction of the graft chains onto poly‐(R)‐2 resulted in the main chain of the polymer having an M‐helical morphology. The complexation of copolymers poly‐[(R)‐2‐co‐(S)‐2] by the capsule gave rise to the unique chiral amplification known as the majority‐rules effect.
Tsunoda, Yuta; Takatsuka, Mei; Sekiya, Ryo; Haino Takeharu, Angew. Chem. Int. Ed.2017,56, 2613-2618
●Site-Selective Encapsulation of Different Anions in a Quadruply Interlocked Dimer
Interlocked dimer 2, which is composed of two physically interlocked monomers 1, has three cavities (cavity A × 2 and cavity B × 1) and can encapsulate three anions, such as NO3− and BF4−, one anion per cavity. There are six possible encapsulation patterns, A–F; two (A and F) contain only one kind of anion and the others (B–E) contain both NO3− and BF4− at the same time with different ratios and with different positions. Anion competition experiments showed that in addition to F, which encapsulates three NO3− ions, C, in which NO3− and BF4− ions are captured in cavities A and cavity B, respectively, was selectively formed. Detailed investigations have revealed that B–E were formed by dimerization, but three of the four were subjected to anion exchange and converged into C or F. This selective formation can be explained by the fact that NO3− is a better anion template than BF4−, as well as the molecular structure of the interlocked dimer; cavities A are surrounded by four bridging ligands and can be accessed by free anions, whereas no space available for anion exchange is present around cavity B because this cavity is surrounded by eight bridging ligands. Therefore, the BF4− ions in cavities A are expelled by free NO3−, but the BF4− ion in cavity B is not, resulting in the selection of C and F. We have found that the volume of the cavity influenced anion recognition. New interlocked dimer 3, which has smaller cavities than those of 2, captured three NO3− ions to form F, whereas only a small amount of an interlocked dimer that contains both NO3− and BF4− was formed.
Sekiya Ryo; Fukuda Morihiko; Kuroda Reiko, Org. Biomol. Chem.2017,15, 4328-4335
●Vanadium(V) Complexes of Some Bidentate Hydrazone Ligands and Their Bromoperoxidase Activity
Dinuclear methoxy bridged complexes of vanadium, [VO(µ-OMe)(OMe)(L)]2 (1–3) have been synthesized from the reaction of VOSO4·H2O with triethylamine and the respective hydrazone ligand. The compounds have been characterized by spectroscopic methods and determination of single crystal X-ray structure of one of them (1). DFT and TD-DFT calculations were used to understand the electronic structures of the complexes and their electronic spectra respectively. Though the dimeric complexes are stable in the solid state, the ESI-MS spectra as well as 1H NMR spectra of the complexes suggest that in solution the monomeric forms of the complexes are the major species. The V(V) complexes in DMF were used to catalyze the oxidative bromination of salicylaldehyde, in aqueous H2O2/KBr in the presence of HClO4 at room temperature. The complexes show exceptionally high bromoperoxidase activity with salicylaldehyde as a model substrate to produce 5-bromo salicylaldehyde in good yield and high TOF and TON. Therefore, these complexes behave as functional models of vanadate dependent bromoperoxidase enzyme.
Adak, Piyali; Ghosh, Bipinbihari; Pakhira, Bholanath; Sekiya, Ryo; Kuroda, Reiko, Polyhedron,2017,127, 135-143
DOI:10.1016/j.poly.2017.01.054
●Light-harvesting organogel based on tris(phenylisoxazolyl)benzene
A novel light-harvesting organogel based on tris(phenylisoxazolyl)benzene, 1, was developed. A pyrene derivative, 2, possessing four phenylisoxazole substituents, was synthesised as an energy acceptor. The electronically excited-state energy of 1 was transferred to a small portion of 2 in the gel state, whereas the photo-induced energy transfer was not observed in solution. The coassembled structures of 1 and 2, formed in the decalin gel, exhibited light-harvesting behaviour. The study on the fluorescence quantum yield revealed that one molecule of 2 accepts the excited-state energy from approximately eight molecules of 1. Time-resolved fluorescence decay experiments revealed that the fluorescence resonance energy transfer is dominant in the energy transfer process in the gel state.
Ikeda, Toshiaki; Ueda, Yuko; Komori, Naomitsu; Abe, Manabu; Haino, Takeharu, Supramol. Chem., 2017, 29, 471-476
DOI:10.1080/10610278.2016.1268692
2016
●Allostery in Guest Binding of Rim-to-Rim-Connected Homoditopic Biscavitands

Rim‐to‐rim‐connected phosphonate biscavitands 1a and 1b were synthesized. Crystal structure analysis and variable‐temperature NMR spectroscopy studies reveal that 1a is more rigid in conformation than 1b. Biscavitands 1a and 1b bind cationic guests G1–G3 through hydrogen bonding and CH–π interactions, demonstrating positive and negative allosteric effects, respectively. The allosteric effects in guest binding are associated with the conformational flexibilities of 1a and 1b. The positive allosteric effect of 1a is directed by the tightly connected cavities, in which information of the first guest binding is transferred to the remaining cavity, which becomes preorganized. In contrast, a slightly negative allosteric effect is found in more‐flexible 1b.
Shimoyama, Daisuke; Yamada, Hitomi; Ikeda, Toshiaki; Sekiya, Ryo; Haino, Takeharu, Eur. J. Org. Chem., 2016, 3300-3303(Cover Picture)
●Synthesis and Properties of Novel Optically Active Platinum-containing Poly(phenyleneethynylene)s
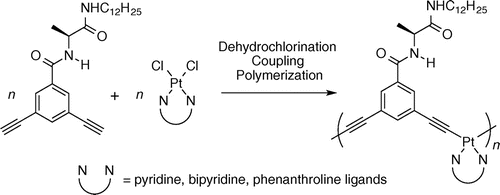
The dehydrochlorination coupling polymerization of N-(3,5-diethynylbenzoyl)-l-alanine dodecylamide 1 with platinum (Pt) chlorides having pyridine, bipyridine, and phenanthroline ligands 2a–2d gave novel optically active poly(phenyleneethynylene)s [poly(1-2a)–poly(1-2d)] bearing Pt in the main chains. The Z-average diameters of the polymers ranged from 453 to 1081 nm. Poly(1-2a) exhibited CD signals assignable to aggregates, and formed regulated twisted structures with height of 540 ± 70 nm and pitch of 62 ± 6 nm, which were confirmed by AFM measurements.
Otaki, Yoshinori; Marumoto, Manabu; Miyagi, Yu; Hieran, Takehiro; Haino, Takeharu; Sanda, Fumio, Chem. Lett.2016,45, 937-939
●Cooperative Self-Assembly of Carbazole Derivatives Driven by Multiple Dipole-Dipole Interactions
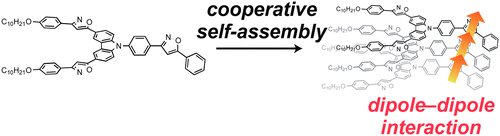
Carbazole possessing phenylisoxazoles self-assembled in a cooperative manner in decalin. X-ray crystal structure analysis revealed that the isoxazole dipoles align in a head-to-tail fashion. DFT calculations suggested that the linear array of dipoles induced the polarization of each dipole, leading to an increase in dipole–dipole interactions. This dipole polarization resulted in cooperative assembly.
Ikeda, Toshiaki; Iijima, Tatsuya; Sekiya, Ryo; Takahashi, Osamu; Haino, Takeharu, J. Org. Chem., 2016, 81, 6832-6837
●Synthesis of a Pentacene-Type Silaborin via Double Dehydrogenative Cyclization of 1,4-Diboryl-2,5-disilylbenzene
A new pentacene‐type silaborin, in which three benzene rings are bridged by silicon and boron atoms, has been synthesized and characterized by using NMR spectroscopy and X‐ray crystallographic analysis. The precursor, 1,4‐bis(dimesitylboryl)‐2,5‐bis(phenylsilyl)benzene (4), was prepared by stepwise introduction of a silyl group and a boryl group to a benzene ring starting from 1,4‐dibromobenzene. Double cyclization of 4 proceeds by a H‐Mes exchange and a B‐H/C‐H dehydrogenative condensation to afford pentacene‐type silaborin 5. X‐ray crystal structure analysis reveals that 5 adopts a bent structure rather than a planar one. UV/Vis spectra and DFT calculations for 5 reveal a lowering of the LUMO energy level compared with corresponding anthracene‐type 3.
Hirofuji, Tatsuya; Ikeda, Toshiaki; Haino, Takeharu; Yamamoto, Yohsuke; Kawachi, Atsushi, Chem. Eur. J., 2016, 22, 9734-9738.
●Chemical Functionalisation and Photoluminescence of Graphene Quantum Dots
Chemical modification of graphene quantum dots (GQDs) can influence their physical and chemical properties; hence, the investigation of the effect of organic functional groups on GQDs is of importance for developing GQD–organic hybrid materials. Three peripherally functionalised GQDs having a third‐generation dendritic wedge (GQD‐2), long alkyl chains (GQD‐3) and a polyhedral oligomeric silsesquioxane group (GQD‐4) were prepared by the CuI‐catalysed Huisgen cycloaddition reaction of GQD‐1 with organic azides. Cyclic voltammetry indicated that reduction occurred on the surfaces of GQD‐1–4 and on the five‐membered imide rings at the periphery, and this suggested that the functional groups distort the periphery by steric interactions between neighbouring functional groups. The HOMO–LUMO bandgaps of GQD‐1–4 were estimated to be approximately 2 eV, and their low‐lying LUMO levels (<−3.9 eV) were lower than that of phenyl‐C61‐butyric acid methyl ester, an n‐type organic semiconductor. The solubility of GQD‐1–4 in organic solvents depends on the functional groups present. The functional groups likely cover the surfaces and periphery of the GQDs, and thus increase their affinity for solvent and avoid precipitation. Similar to GQD‐2, both GQD‐3 and GQD‐4 emitted white light upon excitation at 360 nm. Size‐exclusion chromatography demonstrated that white‐light emission originates from the coexistence of differently sized GQDs that have different photoluminescence emission wavelengths.
Sekiya, Ryo; Uemura, Yuichiro; Naito, Hiroyoshi; Naka, Kensuke; Haino, Takeharu, Chem. Eur. J., 2016, 22, 8198-8206.
●Hydrogen-bonded hexameric cluster of benzyl alchol in the solid state polymeric organization of p-tert-Butylcalix[5]arene

An uncommon hydrogen-bonded hexameric cluster of benzyl alcohol (2) was formed in hydrophobic space in the layered organisation of the one-dimensional polymeric zigzag array of 1 in the solid state. The hexameric cluster adopted a distorted cyclohexane-like (12) O–H⋯O hydrogen bond network. The formation of the hexameric cluster was quite sensitive to a tiny structural difference of guests; phenylethylalcohol (3), phenol (4), benzyl amine (5) and aniline (6) did not form any hexameric cluster. The packing coefficient of 0.53 suggested that the hexameric cluster nicely filled the hydrophobic space, which most likely resulted in the effective van der Waals contacts that stabilised the supramolecular organisation composed of the hexameric cluster and the polymeric array of 1 in the solid state.
Kajiki, Yasunori; Sekiya, Ryo; Haino, Takeharu, Supramol. Chem.2016,28, 444-449
DOI:10.1080/10610278.2015.1117084
●Photoresponsive Toroidal Nanostructure Formed by Self-assembly of Azobenzene-Functionalized Tris(phenylisoxazolyl)benzene
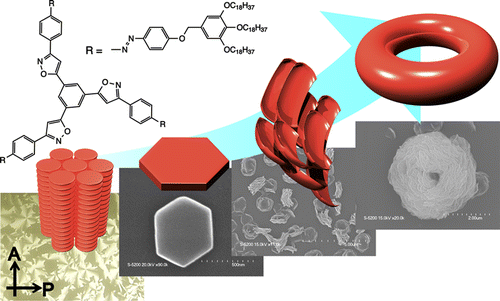
The self-assembly of tris(phenylisoxazolyl)benzene 1b with photochemically addressable azobenzene moieties produced toroidal nanostructures, the formation and dissociation of which were reversibly regulated upon photoirradiation. 1b displayed a mesogenic behavior. In the solution, the stacked assemblies along with their C3 axes were formed. In the mesophase, two molecules of 1b most likely adopted the antiparallel arrangement to stabilize the columnar organization. This assembling behavior most likely triggered the development of the supramolecular toroidal nanostructures.
Adachi, Hiroaki; Hirai, Yuko; Ikeda, Toshiaki; Maeda, Makoto; Hori, Ryo; Kutsumizu, Shoichi; Haino, Takeharu, Org. Lett., 2016, 18, 924-927.
DOI:10.1021/acs.orglett.5b03622
●Frozen Dissymmetric Cavities in Resorcinarene-Based Coordination Capsules
By introducing slight structural modifications to a D4‐symmetric coordination capsule, we succeeded in isolating the nearly enantiopure capsules (P)‐ and (M)‐2 a(BF4)4. Chiral guest, dibenzyl 4,4′‐diacetoxy‐6,6′‐dimethyl‐[1,1′‐biphenyl]‐2,2′‐dicarboxylate (3) was encapsulated within the dissymmetric cavity of 2 a(BF4)4, resulting in a high diastereoselectivity of >99 % de. The encapsulated guest was successfully removed from the complex without racemization through precipitation of the empty capsule. CD spectra confirmed that the chirality of the capsule was maintained in THF and 1,4‐dioxane for long periods, whereas a small amount of acetonitrile accelerated racemization of the empty capsule. The activation parameters of the racemization reaction were determined in dichloromethane and 1,2‐dichloroethane, resulting in positive enthalpic contributions and large negative entropic contributions, respectively. Accordingly, the racemization fits a first‐order kinetic model. Mechanically coupled Cu+‐2,2′‐bipyridine coordination centers were responsible for the high‐energy barrier of racemization and led to the unique chiral memory of the dissymmetric cavity, which was turned off by the addition of acetonitrile.
Imamura, Taisuke; Maehara, Takeshi; Sekiya, Ryo; Haino, Takeharu, Chem. Eur. J., 2016, 22, 3250-3254
●Induced-Fit Molecular Recognition of Alkyl Chains in p-tert-Butylcalix[5]arene in the Solid state
p-tert-Butylcalix[5]arene 1 recognized conformationally flexible aliphatic hydrocarbons (hexane 2, heptane 3, octane 4, nonane 5, decane 6, undecane 7, and dodecane 8) and monoalkyl benzenes (toluene 10, ethylbenzene 11, propylbenzene 12, butylbenzene 13, hexylbenzene 14, and octylbenzne 15) to form host–guest complexes. X-ray diffraction study has revealed that the alkyl chains of 11–15 were selectively recognized by 1, whereas host 1 recognized the aromatic ring of 10. The alkyl chain termini of 3, 4, 6–8, 11–13, and 15 adopted unusual folded conformations in the cavity. M06-2X/6-31G(d,p) level of calculations revealed that the mutual induced-fit shape adjustments between the calix[5]arene cavity and the flexible guests play a key role in maximizing the host–guest interactions directed by the C(sp3)–H/π interactions, leading to the unusual conformations of the alkyl chain termini of the guests and the selective binding of the alkyl chains in the solid state.
Kajiki, Yasunori; Sekiya, Ryo; Yamasaki, Yutaro; Uemura, Yuichiro; Haino, Takeharu, Bull. Chem. Soc. Jpn.2016,89, 220-225 (SelectedPaper)
●Synthesis of Linear [5]Catenanes via Olefin Metathesis Dimerization of Pseudorotaxanes Composed of a [2]Catenane and a Secondary Ammonium Salt
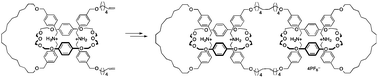
[5]Catenanes were synthesized by olefin metathesis dimerization. The reaction of pseudorotaxanes, which were derived from a [2]catenane and one equivalent of an ammonium salt bearing two terminal olefins in dichloromethane, with a catalytic amount of Grubbs catalyst afforded linear [5]catenanes in 12% yield. Intermolecular and intramolecular olefin metathesis reactions were controlled by the length of the alkyl chain of the ammonium salts.
Iwamoto, Hajime; Tafuku, Shinji; Sato, Yoshihiko; Takizawa, Wataru; Katagiri, Wataru; Tayama, Eiji; Hasegawa, Eietsu; Fukazawa, Yoshimasa; Haino, Takeharu, Chem. Commun., 2016, 52, 319-322
●Solvent-induced Emission of Organogels Based on Tris(phenylisoxazolyl)benzene
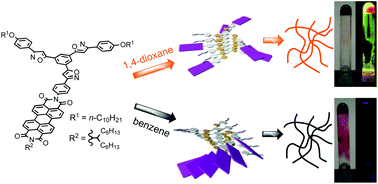
Luminescent organogels based on tris(phenylisoxazolyl)benzene possessing perylenebisimide 1 were synthesized. The emission properties of the gels varied depending on the solvent properties: 1,4-dioxane gel was highly emissive, pyridine gel was moderately emissive, and benzene gel was non-emissive.
Ikeda, Toshiaki; Masuda, Tetsuya; Takayama, Midori; Adachi, Hiroaki; Haino, Takeharu, Org. Biomol. Chem.2016,14, 36-39(Cover Picture)
2015
●Supramolecular Porphyrin Copolymer Assembled through Host-Guest Interactions and Metal-Ligand Coordination

Bisporphyrin cleft molecule 1 Zn possessing a guest moiety assembled to form supramolecular polymers through host–guest interactions. Bispyridine cross‐linkers created interchain connections among the supramolecular polymers to form networked polymers in solution. Solution viscometry confirmed that the cross‐linked supramolecular polymers were highly entangled. Frequency‐dependent linear viscoelastic spectroscopy revealed that the supramolecular polymers generated well‐entangled solutions with associating and networking polymers, whereas the solid‐like aggregates moved individually without breaking and reforming structures below the transition temperature of 9.6 °C. Morphological transition of the supramolecular polymers was evidenced by AFM images; the non‐cross‐linked polymer resulted in wide‐spread thin networks, while the cross‐linked networks produced thicker worm‐like nanostructures. The supramolecular networks gelled in 1,1,2,2‐tetrachloroethane, and an elastic free‐standing film was fabricated with a Young’s modulus of 1 GPa.
Kinjo, Kanashi; Hirao, Takehiro; Kihara, Shin-ichi; Katsumoto, Yukiteru; Haino, Takeharu, Angew. Chem. Int. Ed., 2015, 54, 14830-14834
●Synthesis of Optically Active Conjugated Polymers Containing Platinum in the Main Chain: Control of the Higher-Order Structures by Substituents and Solvents
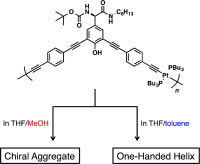
The Sonogashira–Hagihara coupling polymerization of d‐hydroxyphenylglycine‐derived diiodo monomers 1–4 and platinum‐containing diethynyl monomer 5 gave the corresponding polymers [poly(1–5)–(2–5)] with number‐average molecular weights of 19,000–25,000 quantitatively. The polymers were soluble in CHCl3, CH2Cl2, THF, and DMF. CD and UV–vis spectroscopic analysis revealed that amide‐substituted polymers [poly(1–5) and poly(2–5)] formed chiral higher‐order structures in solution, while ester‐substituted polymers [poly(3–5) and poly(4–5)] did not. Poly(1–5) formed one‐handed helices in THF/toluene mixtures, while it formed chiral aggregates in THF/MeOH mixtures. Poly(1–5) emitted fluorescence with quantum yields ranging from 0.8 to 1.3%. The polymers usually aggregated in the solid state.
Miyagi, Yu; Hirao, Takehiro; Haino, Takeharu; Sanda, Fumio, J. Polym. Sci. A Polym. Chem., 2015, 53, 2452-2461
●Supramolecular Polymerization Engineered with Molecular Recognition
Supramolecular polymeric assemblies represent an emerging, promising class of molecular assemblies with enormous versatility compared with their covalent polymeric counterparts. Although a large number of host–guest motifs have been produced over the history of supramolecular chemistry, only a limited number of recognition motifs have been utilized as supramolecular connections in polymeric assemblies. This account describes the molecular recognition of host molecules based on calix[5]arene and bisporphyrin that demonstrate unique guest encapsulations; subsequently, these host–guest motifs are applied to the synthesis of supramolecular polymers that display polymer‐like properties in solution and solid states. In addition, new bisresorcinarenes are developed to form supramolecular polymers that are connected via a rim‐to‐rim hydrogen‐bonded dimeric structure, which is composed of two resorcinarene moieties.
Haino, Takeharu, Chem. Rec., 2015, 15, 837-853
●New Insights into Metal Ion-crown Ether Complexes Revealed by SEIRA Spectroscopy

We demonstrate a powerful spectroscopic technique, surface-enhanced infrared absorption (SEIRA) spectroscopy, not only for detecting host–guest complexes in solution but also for examining the relationship between the guest selectivity, complex structure, and solvent effect. We synthesize thiol derivatives of 15-crown-5 and 18-crown-6 [2-(6-mercaptohexyloxy)methyl-15-crown-5 (15C5-C1OC6-SH) and 2-(6-mercaptohexyloxy)methyl-18-crown-6 (18C6-C1OC6-SH)], which are adsorbed on gold surfaces through S–Au bonds. The IR difference spectra of the M+·15C5-C1OC6 (M = Li, Na, K, Rb, and Cs) complexes on gold are observed using aqueous solutions of MCl by SEIRA spectroscopy. The spectra show a noticeable change in the C–O stretching vibration at around 1100 cm−1. The spectral patterns of M+·15C5-C1OC6 are similar for Li+ and Na+, and for K+, Rb+, and Cs+; the interaction between the metal ions and 15C5-C1OC6 changes drastically between Na+ and K+ in the series of alkali metal ions. On the other hand, the equilibrium constant for complex formation determined by the IR intensity shows clear preference for Na+ ions. We also observe the IR difference spectra of M+·18C6-C1OC6 in methanol and compare them with those in water. The spectral patterns in methanol are almost the same as those in water, but the equilibrium constant in methanol does not show preference for any ion, different from the K+ preference in water. From these findings we attribute the origin of the ion selectivity of 15C5 and 18C6 in solution to the interaction between the metal ions and the crown ethers in the complexes or the solvation energy of free ions. In the case of 15C5-C1OC6 in water, the preference of Na+ over K+, Rb+, and Cs+ can be attributed to the strength of the interaction or the size matching between metal ions and 15C5-C1OC6; the Na+ selectivity over Li+ ions is dominated by the solvation energy of free ions. For 18C6-C1OC6 in methanol, the equilibrium constant for complex formation becomes much bigger in methanol than that in water and loses the selectivity in methanol, because the solvation energy in methanol is fairly smaller than that in water, predominating the contribution from the strength of the interaction between metal ions and 18C6-C1OC6. The IR spectra measured by SEIRA spectroscopy are quite sensitive to properties of host–guest complexes such as the intermolecular interaction, the structure, and the orientation against the gold surface. However, the evidence for guest selectivity emerges primarily in the intensity of the spectra, rather than band positions or spectral patterns in the IR spectra.
Inokuchi, Yoshiya;Ebata, Takayuki; Ikeda, Toshiaki; Haino, Takeharu; Kimura, Tetsunari; Guo, Hao; Furutani, Yuji, New J. Chem., 2015, 39, 8673-8680
●UV Photodissociation Spectroscopy of Cryogenically Cooled Gas Phase Host-guest Complex Ions of Crown Ethers

The geometric and electronic structures of cold host–guest complex ions of crown ethers (CEs) in the gas phase have been investigated by ultraviolet (UV) fragmentation spectroscopy. As host CEs, we chose 15-crown-5 (15C5), 18-crown-6 (18C6), 24-crown-8 (24C8), and dibenzo-24-crown-8 (DB24C8), and as guests protonated-aniline (aniline·H+) and protonated-dibenzylamine (dBAM·H+) were chosen. The ions generated by an electrospray ionization (ESI) source were cooled in a quadrupole ion-trap (QIT) using a cryogenic cooler, and UV spectra were obtained by UV photodissociation (UVPD) spectroscopy. UV spectroscopy was complemented by quantum chemical calculations of the most probable complex structures. The UV spectrum of aniline·H+·CEs is very sensitive to the symmetry of CEs; aniline·H+·18C6 shows a sharp electronic spectrum similar to aniline·H+, while aniline·H+·15C5 shows a very broad structure with poor Franck–Condon factors. In addition, a remarkable cage effect in the fragmentation process after UV excitation was observed in both complex ions. In aniline·H+·CE complexes, the cage effect completely removed the dissociation channels of the aniline·H+ moiety. A large difference in the fragmentation yield between dBAM·H+·18C6 and dBAM·H+·24C8 was observed due to a large barrier for releasing dBAM·H+ from the axis of rotaxane in the latter complex.
Inokuchi, Yoshiya; Haino, Takeharu; Sekiya, Ryo; Morishima, Fumiya; Dedonder, Claude; Feraud, Geraldine; Jouvet, Christophe; Ebata, Takayuki, Phys. Chem. Chem. Phys., 2015, 17, 25925-25934
●Liposome Collapse Resulting from an Allosteric Interaction between 2,6-Dimethyl-beta-Cyclodextrins and Lipids
Although heptakis(2,6-di-O-methyl)-β-cyclodextrin (DMe-β-CDx) has been reported to exhibit higher cytotoxicity than many other cyclodextrins because of the way in which it abstracts cholesterols from liposomes, we have identified another reason for its cytotoxicity based on its interaction with lipids. These interactions exhibited nonlinear sigmoidal responses with Hill coefficient values (n) in the range of 3.0–3.6, which indicated that this phenomenon involves positive allosterism. Furthermore, analysis by mass spectroscopy revealed that the lipid–DMe-β-CDx complexes had stoichiometric ratios in the range of 1 : 1–1 : 4.
Ikeda, Atsushi; Iwata, Noboru; Hino, Shodai; Mae, Tomoya; Tsuchiya, Yuki; Sugikawa, Kouta; Hirao, Takehiro; Haino, Takeharu; Ohara, Kazuaki; Yamaguchi, Kentaro, RSC Adv., 2015, 5, 77746-77754
●Novel Helical Assembly of a Pt(II) Phenylbipyridine Complex Directed by Metal-metal Interaction and Aggregation-induced Circularly Polarized Emission
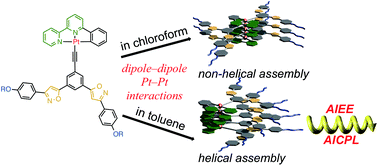
Pt(II) phenylbipyridine complexes possessing bis(phenylisoxazolyl)phenylacetylene ligands self-assembled to form stacked aggregates via Pt–Pt, π–π stacking, and dipole–dipole interactions. The assembled structures were influenced by the solvent properties. Non-helical assemblies found in chloroform displayed metal–metal-to-ligand charge transfer absorption and emission, whereas helical assemblies formed in toluene showed aggregation-induced enhancement of emission and aggregation-induced circularly polarized luminescence. The rates of the association and dissociation of the assemblies were significantly reduced in toluene, and the non-helical structures formed in chloroform were surprisingly memorized.
Ikeda, Toshiaki; Takayama, Midori; Kumar, Jatish; Kawai, Tsuyoshi; Haino, Takeharu, Dalton Trans., 2015, 44, 13156-13162
●Facile Construction of Well-defined Fullerene-dendrimer Supramolecular Nanocomposites for Bioapplications
Well-defined fullerene–dendrimer supramolecular nanocomposites exhibiting uniform size, controlled morphology, high fullerene inclusion efficiency, excellent water solubility, and non-toxicity were facilely fabricated through complexation of carboxyfullerenes with poly(ethylene glycol)-modified poly(amidoamine) dendrimers.
Li, Xiaojie; Watanabe, Yasuo; Yuba, Eiji; Harada, Atsushi; Haino, Takeharu; Kono, Kenji, Chem. Commun., 2015, 51, 2851-2854
●Molecular Recognition of Upper Rim Functionalized Cavitand and Its Unique Dimeric Capsule in the Solid State
Cavitand 1 possesses four 2,2′-bipyridyl pillars on its upper rim that encapsulates small guests, such as nitromethane, acetonitrile, methyl acetate, ethyl acetate, and N-methylacetamide, into a deep cavity to form host–guest complexes in a 1 : 1 ratio. Nitroethane, N,N-dimethylformamide, and N,N-dimethylacetamide were not bound in this manner. A guest-binding study and molecular mechanics calculations revealed that the four 2,2′-bipyridyl pillars of cavitand 1 created a steric boundary that is responsible for selective guest recognition. In the solid state, cavitand 2 formed a unique chiral capsule 22 by π–π stacking interactions between the 2,2′-bipyridyl pillars. A nitromethane molecule wasunusually placed deep inside the cavity, as directed by the multiple hydrogen bonding interactions between the nitromethane oxygen atoms, the C–H bonds of the bridge methylenes and the pillar phenyl groups.
Kobayashi, Mutsumi; Takatsuka, Mei; Sekiya, Ryo; Haino, Takeharu, Org. Biomol. Chem., 2015, 13, 1647-1653(Cover Picture)
●Anomalous Cage Effect of the Excited State Dynamics of Catechol in the 18-Crown-6-Catechol Host-Guest Complex
We determined the number of isomers and their structures for the 18-crown-6 (18C6)–catechol host–guest complex, and examined the effect of the complex formation on the S1 (1ππ*) dynamics of catechol under a supersonically cooled gas phase condition and in cyclohexane solution at room temperature. In the gas phase experiment, UV–UV hole-burning spectra of the 18C6–catechol 1:1 complex indicate that there are three stable isomers. For bare catechol, it has been reported that two adjacent OH groups have an intramolecular hydrogen (H) bond. The IR–UV double resonance spectra show two types of isomers in the 18C6–catechol 1:1 complex; one of the three 18C6–catechol 1:1 isomers has the intramolecular H-bond between the two OH groups, while in the other two isomers the intramolecular H-bond is broken and the two OH groups are H-bonded to oxygen atoms of 18C6. The complex formation with 18C6 substantially elongates the S1 lifetime from 7 ps for bare catechol and 2.0 ns for the catechol–H2O complex to 10.3 ns for the 18C6–catechol 1:1 complex. Density functional theory calculations of the 18C6–catechol 1:1 complex suggest that this elongation is attributed to a larger energy gap between the S1 (1ππ*) and 1πσ* states than that of bare catechol or the catechol–H2O complex. In cyclohexane solution, the enhancement of the fluorescence intensity of catechol was found by adding 18C6, due to the formation of the 18C6–catechol complex in solution, and the complex has a longer S1 lifetime than that of catechol monomer. From the concentration dependence of the fluorescence intensity, we estimated the equilibrium constant K for the 18C6 + catechol ⇄ 18C6–catechol reaction. The obtained value (log K = 2.3) in cyclohexane is comparable to those for alkali metal ions or other molecular ions, indicating that 18C6 efficiently captures catechol in solution. Therefore, 18C6 can be used as a sensitive sensor of catechol derivatives in solution with its high ability of fluorescence enhancement.
Morishima, Fumiya; Kusaka, Ryoji; Haino, Takeharu; Ebata, Takayuk, J. Phys. Chem. B, 2015, 119, 2557-2565
2014
●Conversion from Pillar[5]arene to Pillar[6-15]arenes by Ring Expansion and Encapsulation of C60by Pillar[n]arenes with Nanosize Cavities

Conversion of ring size from pillar[5]arene to pillar[6–15]arenes and isolation of pillar[n]arene homologues (n = 11–13) with known pillar[n]arene homologues (n = 6–10) are demonstrated. Pillar[10]arene formed the most stable host–guest complex with C60 among the pillar[5–14]arenes.
Ogoshi, Tomoki; Ueshima, Naosuke; Sakakibara, Fumiyasu; Yamagishi, Tada-aki; Haino, Takeharu, Org. Lett., 2014, 16, 2896-2899
●Supramolecular Fullerene Polymers and Networks Directed by Molecular Recognition between Calix[5]arene and C60
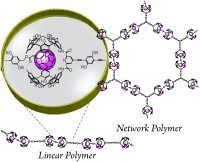
A biscalix[5]arene–C60 supramolecular structure was utilized for the development of supramolecular fullerene polymers. Di‐ and tritopic hosts were developed to generate the linear and network supramolecular polymers through the complexation of a dumbbell‐shaped fullerene. The molecular association between the hosts and the fullerene were carefully studied by using 1H NMR, UV/Vis absorption, and fluorescence spectroscopy. The formation of the supramolecular fullerene polymers and networks was confirmed by diffusion‐ordered 1H NMR spectroscopy (DOSY) and solution viscometry. Upon concentrating the mixtures of di‐ or tritopic hosts and dumbbell‐shaped fullerene in the range of 1.0–10 mmol L−1, the diffusion coefficients of the complexes decreased, and the solution viscosities increased, suggesting that large polymeric assemblies were formed in solution. Scanning electron microscopy (SEM) was used to image the supramolecular fullerene polymers and networks. Atomic force microscopy (AFM) provided insight into the morphology of the supramolecular polymers. A mixture of the homoditopic host and the fullerene resulted in fibers with a height of (1.4±0.1) nm and a width of (5.0±0.8) nm. Interdigitation of the alkyl side chains provided secondary interchain interactions that facilitated supramolecular organization. The homotritopic host generated the supramolecular networks with the dumbbell‐shaped fullerene. Honeycomb sheet‐like structures with many voids were found. The growth of the supramolecular polymers is evidently governed by the shape, dimension, and directionality of the monomers.
Hirao, Takehiro; Tosaka, Masatoshi; Yamago, Shigeru; Haino, Takeharu, Chem. Eur. J., 2014, 20, 16138-16146
●Guest Induced Head-to-tail Columner Assembly of 5,17-Difunctionalized Calix[4]arene
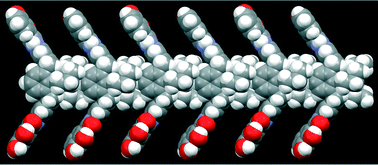
Calix[4]arene possessing two catechol side arms 1 and organic molecules (nPrOH, AcOH, AcOEt, and CH3CN) crystallized to afford cocrystals: 1·(nPrOH)4, 1·(AcOH)4, 1·(AcOEt), and 1·(CH3CN)2. In these cocrystals, calix[4]arene 1 is arranged one-dimensionally, forming head-to-tail columnar structures. The organic guests settle in the residual space between the columnar structures; they are captured by the catechol side arms through hydrogen bonding. In the cocrystal 1·(H2O)2, a continuous zigzag array of 1 is formed instead of the columnar assembly, demonstrating that the organic guests induced the formation of the head-to-tail columnar structures in the crystal packing. The crystalline apohost 1apo was prepared by the desorption of the MeOH guests from the cocrystal 1·(MeOH)4; this compound adsorbed the MeOH vapour, reconstructing the original crystal packing. When 1apo adsorbed the iPrOH vapour, a cocrystal with a crystal structure similar to that of 1·(MeOH)4 was formed, suggesting that 1apo has a crystal structure similar to that of 1·(MeOH)4.
Sekiya, Ryo; Yamasaki, Yutaro; Tada, Wataru; Shio, Hidemi; Haino, Takeharu, CrystEngComm, 2014, 16, 6023-6032
●High Diastereoselection of Dissymmetric Capsule by Chiral Guest Complexation
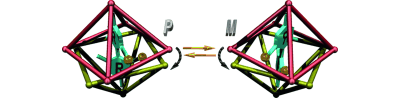
Encapsulation of chiral guests in the dissymmetric capsule 1⋅4 BF4 formed diastereomeric supramolecular complexes G⊂1⋅4 BF4 (G: guest). When chiral guests 2 a–q were encapsulated within the dissymmetric space of the self‐assembled capsule 1⋅4 BF4, circular dichroism (CD) was observed at the absorption bands that are characteristic of the π–π* transition of the bipyridine moiety of the capsule, which suggests that the P and M helicities of the capsule are biased by the chiral guest complexation. The P helicity of diastereomeric complex (S)‐2 l⊂1⋅4 BF4 was determined to be predominant, based on CD exciton coupling theory and DFT calculations. The diastereoselectivity was highly influenced by the ester substituents, such that benzyl ester moieties were good for improving the diastereoselectivity. A diastereomeric excess of 98 % was achieved upon the complexation of 2 j. The relative enthalpic and entropic components for the distereoselectivity were obtained from a van’t Hoff plot. The enthalpic components were linearly correlated with the substituent Hammett parameters (σp+). The electron‐rich benzyl ester moieties generated donor–acceptor π–π stacking interactions with the bipyridine moiety, which resulted in a significant difference in energy between the predominant and subordinate diastereomeric complexes.
Tsunoda, Yuta; Fukuta, Katsunori; Imamura, Taisuke; Sekiya, Ryo; Furuyama, Taniyuki; Kobayashi, Nagao; Haino, Takeharu, Angew. Chem. Int. Ed., 2014, 53, 7243-7247
●White-Light-Emitting Edge-Functionalized Graphene Quantum Dots

Graphene quantum dots (GQDs) have received considerable attention for their potential applications in the development of novel optoelectronic materials. In the generation of optoelectronic devices, the development of GQDs that are regulated in terms of their size and dimensions and are unoxidized at the sp2 surfaces is desired. GQDs functionalized with bulky Fréchet’s dendritic wedges at the GQD periphery were synthesized. The single‐layered, size‐regulated structures of the dendronized GQDs were revealed by atomic force microscopy. The edge‐functionalization of the GQDs led to white‐light emission, which is an uncommon feature.
Sekiya, Ryo; Uemura, Yuichiro; Murakami, Hideki; Haino, Takeharu, Angew. Chem. Int. Ed., 2014, 53, 5619-5623
●Development of Ultraviolet-Ultraviolet Hole-burning Spectroscopy for Cold Gas Phase Ions

A new ultraviolet–ultraviolet hole-burning (UV–UV HB) spectroscopic scheme has been developed for cold gas-phase ions in a quadrupole ion trap (QIT) connected with a time-of-flight (TOF) mass spectrometer. In this method, a pump UV laser generates a population hole for the ions trapped in the cold QIT, and a second UV laser (probe) monitors the population hole for the ions extracted to the field-free region of the TOF mass spectrometer. Here, the neutral fragments generated by the UV dissociation of the ions with the second laser are detected. This UV–UV HB spectroscopy was applied to protonated dibenzylamine and to protonated uracil. Protonated uracil exhibits two strong electronic transitions; one has a band origin at 31760 cm–1 and the other at 39000 cm–1. From the UV–UV HB measurement and quantum chemical calculations, the lower-energy transition is assigned to the enol–keto tautomer and the higher-energy one to the enol–enol tautomer.
Féraud, Géraldine; Dedonder-Lardeux, Claude; Jouvet, Christophe; Inokuchi, Yoshiya; Haino, Takeharu; Sekiya, Ryo; Ebata, Takayuki, J. Phys. Chem. Lett., 2014, 5, 1236-1240
●Formation of Host-Guest Complexes on Gold Surface Investigated by Surface-Enhanced IR Absorption Spectroscopy
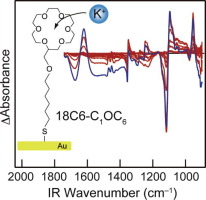
We apply surface-enhanced infrared absorption (SEIRA) spectroscopy to host–guest complexes in liquid phase to examine the structural change in the complex formation. Two thiol derivatives of 18-crown-6 (18C6) are chemisorbed on a gold surface, and aqueous solutions of MCl salts (M = Li, Na, K, Rb, and Cs) are put to form M+·18C6 complexes. Infrared spectra of these complexes in the 900–2000 cm−1 region are obtained by SEIRA spectroscopy. The observed IR spectra show noticeable peaks due to the complex formation, demonstrating that SEIRA spectroscopy will be a powerful method to investigate the structure of host–guest complexes in supramolecular chemistry.
Inokuchi, Yoshiya; Mizuuchi, Takahiro; Ebata, Takayuki; Ikeda, Toshiaki; Haino, Takeharu; Kimura, Tetsunari; Guo, Hao; Furutani, Yuji, Chem. Phys. Lett., 2014, 592, 90-95
DOI:10.1016/j.cplett.2013.12.026
●Self-Assembly of Oligo(phenylisoxazolyl)benzenes Induced by Multiple Dipole-Dipole Interactions
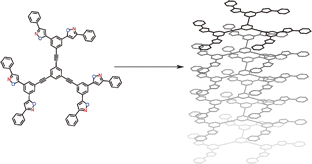
We have demonstrated that oligo(phenylisoxazolyl)benzenes 3–6 formed polymeric assemblies. The intermolecular associations were discussed using thermodynamic parameters. The formation of the polymeric assemblies were confirmed by DOSY and AFM measurements. The large polymeric structures were observed both in solution and in the solid state. The multiple dipole–dipole interactions were crucial for the supramolecular polymerization of the oligo(phenylisoxazolyl)benzenes.
Haino, Takeharu; Ueda, Yuko; Hirao, Takehiro; Ikeda, Toshiaki; Tanaka, Masahiro, Chem. Lett., 2014, 43, 414-416
●Ion-based Assemblies of Planer Anion Complexes and Cationic PtII Complexes
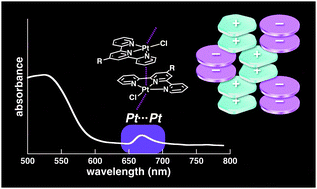
Because the metallophilicity of attractive PtII⋯PtII interactions helps in the fabrication of columnar structures, terpyridine–PtII complexes were used as planar counter cationic species for formation of charge-segregated assemblies using π-conjugated receptor–Cl− complexes as planar anions.
Sekiya, Ryo; TsuTsui, Yusuke; Choi, Wookjin; Sakurai, Tsuneaki; Seki, Shu; Bando, Yuya; Maeda, Hiromitsu, Chem. Commun., 2014, 50, 10615-10618
●Synthesis, Characterization, X-ray Crystal Structurte, DFT Calculations and Catalytic Properties of a Dioxovanadium(V) Complex Derived from Oxamohydrazide and Pyridoxal - A model Complex of Vanadate Dependent Bromoperoxidase)
A vanadium(V) complex with the formula [Et3NH][VVO2(sox-pydx)] with a new tridentate ligand 2-[2-[[3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl]methylene]hydrazinyl]-2-oxoacetamide (soxH-pydxH), obtained by condensation of oxamohydrazide and pyridoxal (one of the forms of vitamin B6), has been synthesized. The compound was characterized by various analytical and spectroscopic methods, and its structure was determined by single-crystal X-ray diffraction technique. Density functional theory (DFT) and time-dependent DFT calculations were used to understand the electronic structure of the complex and nature of the electronic transitions observed in UV–vis spectra. In the complex, vanadium(V) is found to be pentacoordinated with two oxido ligands and a bianionic tridentate ONO-donor ligand. The vanadium center has square-pyramidal geometry with an axial oxido ligand, and the equatorial positions are occupied by another oxido ligand and a phenolato oxygen, an imine nitrogen, and a deprotonated amide oxygen of the hydrazone ligand. A DFT-optimized structure of the complex shows very similar metrical parameters as determined by X-ray crystallography. The O4N coordination environment of vanadium and the hydrogen-bonding abilities of the pendant amide moiety have a strong resemblance with the vanadium center in bromoperoxidase enzyme. Bromination experiments using H2O2 as the oxidizing agent, with model substrate phenol red, and the vanadium complex as a catalyst show a remarkably high value of kcat equal to 26340 h–1. The vanadium compound also efficiently catalyzes bromination of phenol and salicylaldehyde as well as oxidation of benzene to phenol by H2O2.
Das, Chandrima; Adak, Piyali; Mondai, Satyajit; Sekiya, Ryo; Kuroda, Reiko; Gorelsky, Serge; Chattopadhyay, Shyamal, Inorg. Chem., 2014, 53 ,11426-11437
●Heteroleptic Ru(II) Complexes Containing Aroyl Hydrazone and 2,2'-bipyridyl: Synthesis, X-ray Crystal Structure, Electrochemical and DFT Studies
Four heteroleptic Ru(II) complexes [Ru(bpy)2(L)]X (L = bidentate N, O− donor hydrazone ligands, X = ClO4 or PF6) have been synthesized and characterized. Single crystal X-ray structures of two of the complexes are also reported. The complexes show strong absorptions in the 400–600 nm region of the visible radiation, with very similar room temperature emission spectra to that of reference [Ru(bpy)3]X2 but with the lowest energy MLCT (metal to ligand charge transfer) band bathochromically shifted by about 50 nm. Results obtained from both electrochemical experiments and DFT calculations show remarkable differences in the HOMO–LUMO properties of the hydrazone complexes compared to [Ru(bpy)3]X2. TDDFT calculations have been used to understand the nature of the electronic transitions.
Ghosh, Bipinbihari; Naskar, Sumita; Naskar, Subhendu; Espinosa, Arturo; Hau, Sam; Mak, Thomas; Sekiya, Ryo; Kuroda, Reiko, Chattopadhyay, Shyamal, Polyhedron, 2014, 72 , 115-121
DOI:10.1016/j.poly.2014.01.031
2013
●Supramolecular Chemistry: From Host-guest Complexes to Supramolecular Polymers
Combining the concepts of supramolecular chemistry with material science has led to the development of supramolecular polymer chemistry. Although a large number of host-guest motifs have been produced, only a limited number of recognition motifs have been utilized as supramolecular connections within polymeric assemblies. In this account, we describe the molecular recognition of host molecules based on a calix[5]arene and a bisporphyrin, demonstrating unique guest encapsulations; subsequently, these host-guest motifs were applied to the synthesis of supramolecular polymers that display polymer-like properties in both solution and solid states. In addition, we disclose that bisresorcinarenes form supramolecular polymers that are connected via a hydrogen-bonded rim-to-rim dimeric structure, which is composed of two resorcinarene moieties.
Haino, Takeharu, J. Synth. Org. Chem. Jpn., 2013, 71, 1172-1181
DOI:10.5059/yukigoseikyokaishi.71.1172
●Head-to-tail Polymeric Columnar Structure of Calix[4]arene Possessing Catechol Arms in the Solid State
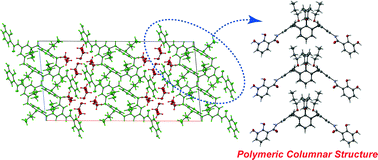
Calix[4]arene 1 cocrystallized with methanol (MeOH), ethanol (EtOH), isopropanol (iPrOH) and 1,4-butanediol (BDO) to afford clathrate compounds 1·(MeOH)4, 1·(EtOH)4, 1·(iPrOH)4, and 1·(BDO)2, respectively. The unique head-to-tail polymeric columnar structures of 1 were found in the solid state.
Sekiya, Ryo; Yamasaki, Yutaro; Katayama, Susumu; Shio, Hidemi; Haino, Takeharu, CrystEngComm, 2013, 15, 8404-8407
●Synthesis of Optically Active Poly(m-phenyleneethynylene-aryleneethynylene)s Bearing Hydroxy Groups and Examination of the Higher Order Structures
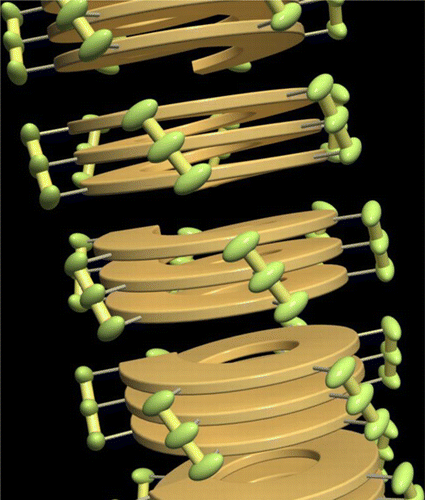
Novel optically active poly(m-phenyleneethynylene–aryleneethynylene)s bearing hydroxy groups with various arylene units {poly[(S)-/(R)-1–3a]–poly[(R)-1–3e] and poly[(S)-2–3a]} were synthesized by the Sonogashira–Hagihara coupling polymerization of 3,5-diiodo-4-hydroxy-C6H4CONHCH(CH3)COXC12H25 [(S)-/(R)-1 (X = O), (S)-2 (X = NH)] with HC≡C–Ar–C≡CH [3a (Ar = 1,4-C6H4), 3b (Ar = 1,4-C6H4-1,4-C6H4−), 3c (Ar = 1,4-C6H4-1,4-C6H4-1,4-C6H4−), 3d (Ar = 2,5-dihexyl-1,4-C6H2), 3e (Ar = 2,5-didodecyl-1,4-C6H2)]. The yields and number-average molecular weights of the polymers were in the ranges 60–94% and 7,000–29,500 with no correlation between the yield and the Mn. Circular dichroism (CD), UV–vis, and fluorescence spectroscopic analyses indicated that poly[(S)-1–3a]–poly[(S)-1–3c] and poly[(S)-2–3a] formed predominantly one-handed helical structures in THF, while poly[(S)-1–3d] and poly[(S)-1–3e] showed no evidence for forming chirally ordered structures. All polymers emitted blue fluorescence. The solution state IR measurement revealed the presence of intramolecular hydrogen bonding between the amide groups at the side chains of poly[(S)-1–2a]. The helical structures and helix-forming abilities of the polymers were analyzed by the molecular mechanics (MM), semiempirical molecular orbital (MO) and density functional theory (DFT) methods. Tube-like structures, presumably formed by perpendicular aggregation of the helical polymers, were observed by atomic force microscopy (AFM).
Sogawa, Hiromitsu; Shiotsuki, Masashi; Hirao, Takehiro; Haino, Takeharu; Sanda, Fumio, Macromolecules, 2013, 46, 8161-8170
●Size- and Orientation-Selective Encapsulation of C70by Cycloparaphenylenes

The size‐ and orientation‐selective formation of the shortest‐possible C70 peapod in solution and in the solid state by using the shortest structural unit of an “armchair” carbon nanotube (CNT), cycloparaphenylene (CPP), has been studied. [10]CPP and [11]CPP exothermically formed 1:1 complexes with C70, thereby giving the resulting peapods. A van′t Hoff plot analysis revealed that the formation of these complexes in 1,2‐dichlorobenzene was mainly driven by entropy, whereas the theoretical calculations suggested that the formation of the complex in the gas phase was predominantly driven by enthalpy. C70 was found to exist in two distinct orientations inside the CPP cavity, namely “lying” and “standing”, depending on the specific size of the CPP. The theoretical calculations and the X‐ray crystallographic analysis revealed that the interactions between [10]CPP and the short axis of C70 in its lying orientation were isotropic and similar to those observed between [10]CPP and C60. However, the interactions between [11]CPP and C70 in its standing orientation were anisotropic, thereby involving the radial deformation of [11]CPP into an ellipsoidal shape. This “induced fit” maximized the van der Waals interactions with the long axis of C70. Theoretical calculations revealed that the deformation occurred readily with low energy loss, thus suggesting that CPPs are highly radially elastic molecules. These results also indicate that the same type of radial deformation should occur in CNT peapods that encapsulate anisotropic fullerenes.
Iwamoto, Takahiro; Watanabe, Yoshiki; Takaya, Hikaru; Haino, Takeharu; Yasuda, Nobuhiro; Yamago, Shigeru, Chem. Eur. J., 2013, 19, 14061-1406
●超分子化学を用いる分子配列構造の制御
灰野岳晴, Organometallic News, 2013, 2, 54-60
●Selective Synthesis of [2]- and [3]Catenane Tuned by Ring Size and Concentration
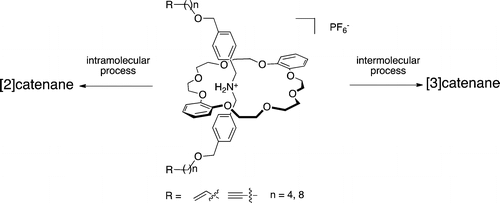
The syntheses of [2]- and [3]catenanes by olefin metathesis and oxidative acetylide coupling have been studied in detail. Pseudorotaxanes that were obtained by mixing crown ether and ammonium salts containing two terminal reactive end-groups were converted to [2]- and [3]catenane. Their yields were influenced not only by the chain length of the ammonium salts but also by the concentration of the crown ether and the ammonium salts. The strain energies of [2]catenane were responsible for the formation of [2]catenane.
Iwamoto, Hajime; Takizawa, Wataru; Itoh, Koji; Hagiwara, Tatsuya; Tayama, Eiji; Hasegawa, Eietsu; Haino, Takeharu, J. Org. Chem., 2013, 78, 5205-5217
●Photoresponsive Two-Component Organogelators based on Trisphenylisoxazolylbenzene

Photochromic tris(phenylisoxazolyl)benzene 1 and bispyridine derivatives 2a–e were mixed in a certain ratio to generate stable gels in benzyl alcohol, 4-methoxybenzyl alcohol, and aniline. Supramolecular assembly of 1 in solution was confirmed by 1H NMR study. The Tgel value was saturated in a 2 : 3 ratio of 1 and 2c. The intermolecular hydrogen bonds OH⋯N and salt bridge O−⋯H–N+ between 1 and 2c coexisted evidently, and these hydrogen bonds contributed to the stabilization of the gel networks. The lengths of alkyl chains of 2a–e governed the stabilities of the gels. The gel formations were driven by the morphological transition of 1 before and after the addition of 2a–e. Mixtures of 1 and 2a–e led to the well developed fibrillar networks, generating a lot of voids that are responsible for immobilizing solvent molecules. When the benzyl alcohol gel was irradiated at 360 nm, the gel turned to the sol. The sol was reversed to the gel by warming. This gel-to-sol phase transition was completely reversible.
Haino, Takeharu; Hirai, Yuko; Ikeda, Toshiaki; Saito, Hiroshi, Org. Biomol. Chem., 2013, 11, 4164-4170(Cover Picture)
●Molecular-recognition-directed formation of supramolecular polymers
Haino, Takeharu, Polym. J., 2013, 45, 363-383